
| Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
| A | ���������Ӳ㣬K��M�������֮�͵���L������� |
| B | �������н�������ǿ |
| C | �����µ���Ϊ˫ԭ�ӷ��ӣ��⻯���ˮ��Һ�ʼ��� |
| D | Ԫ�����������+7�� |
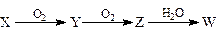
 ...........................................................��1�֣�
...........................................................��1�֣� ............................................................��1�֣�
............................................................��1�֣� ��.��2�֣�
��.��2�֣� ............................................................................��1�֣�
............................................................................��1�֣�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| | �� | �� | �� | �� | �� | �� |
| ԭ�Ӱ뾶��10��10m�� | 1.30 | 0.82 | 0.99 | 1.11 | 0.90 | 1.18 |
| �����ͻ��ϼ� | +2 | +3 | +7 | +4 | +2 | +3 |
| | | ��1 | ��4 | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

| ��A | ��A | IIIA | ��A | ��A | ��A | ��A | 0 | ||||||
| 2 | | | | �� | �� | | �� | | ||||||
| 3 | | �� | �� | | | �� | �� | �� | ||||||
| 4 | �� | | | | | | �� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��H3O+��NH4+��Na+��HF | B��OH-��F-��O2-��Ne |
| C��CH4��NH3��H2O��HF | D��O2-��F-��Mg2+��Al3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��X2Y | B��X2Y2 | C��XY2 | D��XY3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������: HClO4>H2SO4>H3PO4>H2SiO4 | B���ȶ���:HI> HBr>HCl>HF |
| C�����뾶:Na+>K+>Cl->S2- | D��������: K > Na > Mg > Al |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com