

| A����98%��Ũ���������ܽ����õ�4.5 mol·L-1��ϡ���ᣬֻ��3�ֲ������� |
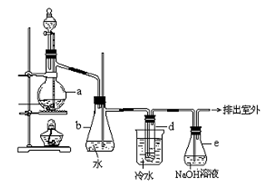
| B�������Ŀ���ǽ���Һ���е�Fe2+���ת����Fe3+��ʵ�����������Һ����ͨ������������ |
| C������Cu(OH)2���CuOҲ�ɵ�����ҺpH����Ӱ��ʵ���� |
| D�������֮���ʵ������������ܼ��ᾧ�������������������������ƾ��ơ������ǡ����ż� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ʵ������ | ʵ����쳣��� | ԭ����� |
| A | �Ʊ�Fe(OH)2 | �۲첻����ɫ���� | ����ԭ���е�Fe2����������δ�������� |
| B | �����ᾧ | ���������� | �ƾ��Ƶ�о�����ȵ�������ײ���������ʣ����Һ��ʱ�������� |
| C | ����ˮ��CCl4 | ��Һ©���������²�Һ���������� | û��װ©�������Ӱε��������ϰ�����©���ڲ����С��û�ж��� |
| D | ��ȼ����ȥCO2�е�CO���� | ����ȼ | CO���Ż��ϸ� |
�鿴�𰸺ͽ���>>
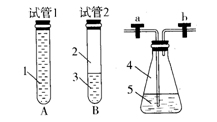
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A������ | B���ռ� | C������ | D�������� (E) KHSO4 |

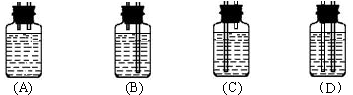
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| �ζ����� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25��00 | 1��02 | 21��03 |
| 2 | 25��00 | 2��00 | 21��99 |
| 3 | 25��00 | 0��20 | 20��20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| �ⶨ��� | ������Һ�����/mL | ���������Һ�����/mL | |
| �ζ�ǰ | �ζ��� | ||
| 1 | 20.00 | 0.50 | 20.78 |
| 2 | 20.00 | 1.20 | 21.32 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
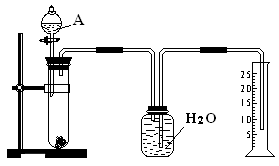
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ������Һ25.00ml����������0.1%��A��Һ��ָʾ����������Ũ��Ϊ0.002000mol/L�������������Һ�ζ�������¼���������������Һ�������
������Һ25.00ml����������0.1%��A��Һ��ָʾ����������Ũ��Ϊ0.002000mol/L�������������Һ�ζ�������¼���������������Һ�������| ��� | �ζ�ǰ/mL | �ζ���/mL |
| �� | 1.35 | 19.40 |
| �� | 1.05 | 19.00 |
| �� | 1.58 | 20. 91 91 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com