
| CH3COOH | H2CO3 | H2S | H3PO4 |
| 1.8��10-5 | K1=4.3��10-7 K2=5.6��10-11 | K1=9.1��10-8 K2=1.1��10-12 | K1=7.5��10-3 K2=6.2��10-8 K3=2.2��10-13 |
���� ��1������ƽ�ⳣ��Խ�������Խǿ��ԽС������Խ����
��2��������ͬ������������������ã�
��3����ͬ�����£���ĵ���ƽ�ⳣ��ԽС����Խ�ѵ��룬�����Խ����������ӣ�
��4��CH3COONa��Һ�д��������ˮ���Լ��ԣ����������Ũ�ȼ�С��
��5������ǿ���������ԭ������Kֵ��������Ƶ�KֵС���ᣬ�ݴ��ж�
��� �⣺��1������ƽ�ⳣ��Խ�������Խǿ��ԽС������Խ�������ݱ���֪��������ǿ����H3PO4���������� HPO42-��
�ʴ�Ϊ��H3PO4��HPO42-��
��2����Ԫ����ֲ����룬��һ������̶���ڶ��������������μ�С��ԭ������һ�����������H+����һ���������������ã�
�ʴ�Ϊ��һ�����������H+�Զ����������������ã�
��3����ͬ�����£���ĵ���ƽ�ⳣ��ԽС����Խ�ѵ��룬�����Խ����������ӣ�����ͬŨ�ȵ�CH3COO-��HCO3-��CO32-��S2-���H+��������ǿ������˳��ΪS2-��CO32-��HCO3-��CH3COO-��
�ʴ�Ϊ��S2-��CO32-��HCO3-��CH3COO-��
��4��CH3COONa��Һ�д��������ˮ���Լ��ԣ����������Ũ�ȼ�С������Һ������Ũ�ȹ�ϵΪc��Na+����c��CH3COO-����c��OH-����c��H+����
�ʴ�Ϊ��c��Na+����c��CH3COO-����c��OH-����c��H+����
��5������ǿ���������ԭ������Kֵ��������Ƶ�KֵС���ᣬ����H3PO4��K1=7.5��10-3����CH3COOH��K=1.8��10-5������CH3COOH+H2PO4-���ܷ�Ӧ��H2CO3��K1=4.3��10-7����H2S��K1=9.1��10-8������H2CO3+HS-�ܷ�Ӧ��
�ʴ�Ϊ�����ܣ��ܣ�
���� ���⿼��ѧ���Զ�Ԫ����ಽ��������⡢�ε�ˮ��ԭ����Ӧ�á��Լ��ӵ���Ƕ����⸴�ֽⷴӦ����������Ŀ�Ѷ��еȣ�ע�����֪ʶ��������ã������ڿ���ѧ���Ի���֪ʶ��Ӧ�������ͷ���������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �屽��ˮ | B�� | ��������Ҵ� | C�� | ���ͺ�ˮ | D�� | �屽�ͱ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
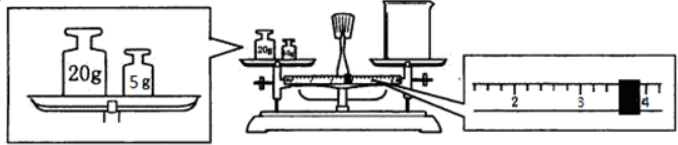
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ����12.5g����NaOHʱ����������������ϣ�NaOH���������� | |
| B�� | ѡ�õ�����ƿ������������ˮ | |
| C�� | ����ʱ���ӿ̶��� | |
| D�� | ����ҡ�Ⱥ�Һ���½����ּ�ˮ���̶��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
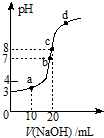 ����ʱ����0.10mol/L��NaOH��Һ�ζ�20.00mL 0.10mol/Lij һԪ��HA��Һ���õζ�������ͼ������˵������ȷ���ǣ�������
����ʱ����0.10mol/L��NaOH��Һ�ζ�20.00mL 0.10mol/Lij һԪ��HA��Һ���õζ�������ͼ������˵������ȷ���ǣ�������| A�� | a��b��c������ʾ��Һ����������ǿ����c���Ӧ����Һ | |
| B�� | ��c����Һ�У�c��H+��+c��HA��=c��OH-�� | |
| C�� | 25�棬HA�ĵ���ƽ�ⳣ��ԼΪ1.0��10-5 | |
| D�� | a��b��c��d�ĵ���ʾ��Һ��ˮ�ĵ���̶�������b���Ӧ����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1��1-���������1��2-�������� | B�� | ���ͼױ� | ||
| C�� | �Ҷ����ͱ����� | D�� | ��ϩ�ͻ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
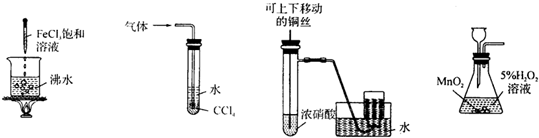
| A�� | �õ����ɫ���� | B�� | ���հ���������ֹ���� | ||
| C�� | �Ʊ����ռ�����NO2���� | D�� | ʵ������O2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com