������Ԫ��A��B��C��D�У�0.5mol AԪ�ص����ӵõ�6.02��10
23�����ӱ���ԭΪ����ԭ�ӣ�0.4g A��������ǡ����100ml 0.2mol/L��������ȫ��Ӧ��Aԭ�Ӻ���������Ŀ��������Ŀ��ȣ�BԪ��ԭ�Ӻ�������������Ŀ�ȵ�һ���1����C
-��AԪ�ص����Ӷ�1�����Ӳ㣬DԪ�ص�ԭ�Ӻ���ڶ���ȵ�һ���2�����ӣ��ش��������⣺
��1��A��B��C��D����Ԫ�ص����Ʒֱ���
��
��
��
��
��2��C
-�Ľṹʾ��ͼΪ
��DԪ�������ڱ��е�λ����
��
��3��Ԫ��D�������̬�⻯��ĵ���ʽΪ
������ӵĽṹ�ص��Ǿ���
�ṹ����һ�������¸��⻯����뵥��C����ȡ����Ӧ�����������ʵ����ĸ��⻯���뵥��C��ϣ���һ�������³�ַ�Ӧ�������������ʵ���������
���û�ѧʽ��д����
��4����ҵ��ұ������A�Ļ�ѧ����ʽΪ
��
��5����ҵ�ϳ��õ���Bұ�����۵Ľ�����д���������͵���B�ڸ����·�Ӧ�Ļ�ѧ����ʽ
���÷�Ӧ����
��Ӧ������ȡ������ȡ�����
��6��д����ҵ����ʯӢ������D��һ�ֵ��������뵼����ϵĻ�ѧ��Ӧ����ʽ��
��7����D�ĵ�������������������ȫȼ�գ����ò���ĽṹʽΪ
��

 ��̼Ԫ��λ��Ԫ�����ڱ��еڶ����ڵڢ�A�壬�ʴ�Ϊ��
��̼Ԫ��λ��Ԫ�����ڱ��еڶ����ڵڢ�A�壬�ʴ�Ϊ�� ���ڶ����ڵڢ�A�壻
���ڶ����ڵڢ�A�壻 ���ռ�ṹΪ���������ͣ���������������ȡ����Ӧ������һ��ȡ��������ȡ��������ȡ��������ȡ��������HCl��������ʵ����ļ�����������ϣ���һ�������³�ַ�Ӧ�������������ʵ���������HCl��
���ռ�ṹΪ���������ͣ���������������ȡ����Ӧ������һ��ȡ��������ȡ��������ȡ��������ȡ��������HCl��������ʵ����ļ�����������ϣ���һ�������³�ַ�Ӧ�������������ʵ���������HCl�� ���������壻HCl��
���������壻HCl��



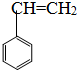 ����CH3-CH=CH-CH3�� ��CH2=CH-CH=CH2����CH��C-CH3����
����CH3-CH=CH-CH3�� ��CH2=CH-CH=CH2����CH��C-CH3����
 ��ͼ��ij��ѧ��ȤС��̽����ͬ�����»�ѧ��ת��Ϊ���ܵ�װ�ã���ش��������⣺
��ͼ��ij��ѧ��ȤС��̽����ͬ�����»�ѧ��ת��Ϊ���ܵ�װ�ã���ش��������⣺