
��1�����ɽ���Ԫ�������γɶ��������磺[Fe(H2NCONH2)6] (NO3)3 [�����������غ���(��)]��Fe(CO)x�ȡ�
�ٻ�̬Fe3+��M������Ų�ʽΪ ��
�������Fe(CO)x������ԭ�Ӽ۵������������ṩ������֮��Ϊ18����x= �� Fe(CO)x�����³�Һ̬���۵�Ϊ��20.5�棬�е�Ϊ103�棬�����ڷǼ����ܼ����ݴ˿��ж�Fe(CO)x�������� ��������ͣ���
 ��2��O��Na�γɵ�һ��ֻ�������Ӽ������ӻ�����ľ����ṹ����ͼ����һ����������Χ���������������Ϊ���㹹�ɵļ�����Ϊ ����֪�þ������ܶ�Ϊ�� g/cm3�������ӵ�����ΪNA�����߳� a = cm�� (�ú�����NA�ļ���ʽ��ʾ)
��2��O��Na�γɵ�һ��ֻ�������Ӽ������ӻ�����ľ����ṹ����ͼ����һ����������Χ���������������Ϊ���㹹�ɵļ�����Ϊ ����֪�þ������ܶ�Ϊ�� g/cm3�������ӵ�����ΪNA�����߳� a = cm�� (�ú�����NA�ļ���ʽ��ʾ)
��3�������йص�˵����ȷ���� ��
A����һ�����ܴ�С��S��P��Si
B���縺��˳��C��N��O��F
C����Ϊ������CaO��KCl�ߣ�����KCl��CaO�۵��
D��SO2��CO2�Ļ�ѧ�������ƣ����ӽṹҲ����ֱ���ͣ���ͬ������SO2���ܽ�ȸ���
E�����Ӿ����У����ۼ�����Խ�÷��Ӿ�����۷е�Խ��
��4��ԭ������С��36��X��Y��Z��W����Ԫ�أ�����X���γɻ�������������Ԫ�أ�Yԭ�ӻ�̬ʱ���������������ڲ��������2����Zԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵĵ��ӣ�W��ԭ������Ϊ29���ش��������⣺
�� Y2X2������Yԭ�ӹ�����ӻ�����Ϊ ��1mol Y2X2���ЦҼ�����ĿΪ ��
�� ������ZX3�ķе�Ȼ�����YX4�ĸߣ�����Ҫԭ���� ��
�� Ԫ��Y��һ����������Ԫ��Z��һ�������ﻥΪ�ȵ����壬Ԫ��Z������������ķ���ʽ�� ��
 ��ҵ����ϵ�д�
��ҵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ�鷽�����е��ǣ��� ��
�� ��A����ϡ�������ˣ���ȥ����ͭ���е�����þ�ۺ�����
���� B������ȡ�ķ����������ͺ�ú��
���� C�����ܽ⡢���˵ķ�������KNO3��NaCl����Ļ����
�� ��D����O2��H2�Ļ����ͨ�����ȵ�����ͭ���Գ�ȥ���е�H2����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ж�ʵ������������������(����)
| A���ý�ͷ�ιܼ�Һʱ�����������Թ��� |
| B������ʢ����ƿ�к�ˮ�ľƾ�������ƿ�ڷ�һЩ���Ƭ |
| C���¶ȼƲ���Һ���в����¶ȵ�ͬʱ������������Һ�� |
| D�����ݻ�Ϊ100 mL����Ͳ��ȡ80 mL��ˮ�Ҵ���ʹҺ����͵����̶�80 mL�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
1)38 gij���۽����Ȼ���(ACl2)�к���0.8mol Cl������ACl2��Ħ��������________��A�����ԭ��������____________��ACl2�Ļ�ѧʽ��__________��
��(2)��������Ϊa��ij���ʵ���Һm g����������Ϊb�ĸ����ʵ���Һn g��Ϻ�������p gˮ���õ�����Һÿ��������Ϊq g�����ʵ���Ũ��Ϊc�������ʵ���Է�������Ϊ����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���������ʵ���Ũ�Ⱦ���ȵ�������Һ��
������ڴ���۰�ˮ����CH3COONa��Һ������˵����ȷ����
A��������ڷֱ�ϡ����ͬ��������Һ��pH����>��
B��������ۻ�Ϻ���Һ�����ԣ����ڳ����� Ka(CH3COOH)= Kb(NH3.NH3)
C����������ѵ����ˮ���ӵ���Ŀ���
D������ܻ��������Һ�����ԣ���c(CH3COO-)+c(OH-)��c(CH3COOH)+c(H+)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����
A��ˮ��һ�ַdz��ȶ��Ļ�������������������
B���Ҵ���������һ������̼ԭ��
C�����Ӿ���ľ�����Խ�����Ӽ�Խǿ
D���縺��:Na�� Al
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڱ�ǰ�����ڵ�Ԫ��a��b��c��d��e��ԭ��������������a�ĺ����������������������ͬ��b�ļ۵��Ӳ��е�δ�ɶԵ�����3����c������������Ϊ���ڲ��������3����d��cͬ�壻e�������ֻ��1�����ӣ����������18�����ӡ��ش��������⣺
��1��b��c��d�е�һ������������ ����Ԫ�ط��ţ���e�ļ۲�����Ų�ʽΪ ��
��2��a������Ԫ���е�һ��Ԫ���γɵĹ��ۻ������У����ӳ������Σ��÷��ӵ�����ԭ�ӵ��ӻ���ʽΪ ������Ԫ���γɵķ����мȺ��м��Թ��ۼ����ֺ��зǼ��Թ��ۼ��Ļ������� ���ѧʽ����
��3����ЩԪ���γɵĺ������У�����е�����ԭ�ӵļ۲���Ӷ���Ϊ3������ ������������νṹ������ �������ѧʽ��
��4��e��c�γɵ�һ�����ӻ�����ľ���ľ����ṹ��ͼ1������e�Ļ��ϼ�Ϊ ��
��5����5��Ԫ���γɵ�һ��������������Ϊ1��1�����ӻ������У������ӳ�������ṹ�������ӳ������İ�����ṹ��ͼ2�����û�������������Ϊ ���������д��ڵĻ�ѧ�������� ��
��6������e�����ṹ��ͼ3����e������ÿ��eԭ����Χ���������eԭ����ĿΪ ��


 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ӷϾɵ�ػᵼ�����صĻ�����Ⱦ��һ�ڷϵ�ؾ���һ�š�ը������ijͬѧ�÷ϸɵ���ڵĺ�ɫ����(���ܺ���MnO2��NH4Cl��ZnCl2������)��������ʵ�飺
��ɫ����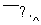 �ܽ�
�ܽ� ����
����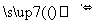 ��������
��������
(1)�������������ж��õ���һ�ֲ���������________________��
(2)�����պ�Ĺ��������Թܣ��μ�˫��ˮ����Ѹ�ٲ���һ��ʹ�����ľ����ȼ�����壬�ɴ��ƶϺ�ɫ�����к���MnO2�����ڸ÷�Ӧ�е�����Ϊ____________��MnO2��һ���������ԣ���д��һ��MnO2���������ķ�Ӧ�Ļ�ѧ����ʽ��______________________________��
(3)��֤����ڵ���Һ�к���NH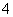 ��������ijһʵ�鷽����¼Ƭ�Σ�����д���пհף�
��������ijһʵ�鷽����¼Ƭ�Σ�����д���пհף�
ʵ����̣�________________________________________________________��
ʵ�������д̼�����ζ�����������
д����ʵ���з�Ӧ�����ӷ���ʽ��_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���б仯��������������Ӧ���ǣ� ����
A��HCl��H2 ������������B��Mg��Mg2������������������C��Cl����AgCl �������� D��CuO��Cu
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com