
����Ŀ��ij������ˮ�к�����̬�ȣ�ͨ������ʵ��ⶨ��Ũ�ȡ�
��ȡˮ��10��0ml����ƿ�У�����10��0ml��KI��Һ(����)�������ķ�ӦΪ��Cl2+2KI��2KCl+I2������ָʾ��2~3�Ρ�
�ڢ�ȡһ�ζ�������������ˮ������ˮϴ��������0.01mol��L-1Na2S2O3��Һ��ϴ��Ȼ��װ��0.01mol��L-1Na2S2O3��Һ��0�̶����ϣ��ų��¶˼����ڵ����ݣ�����Һ����0�̶Ȼ�0�̶���ijһλ�ã����¶�����
�۽���ƿ���ڵζ����½��еζ��������ķ�ӦΪ��I2+2Na2S2O3=2NaI+ 2Na2S4O6 �Իش������ʴ�
��1������ټ����ָʾ����_______________________________��
��2�������Ӧʹ��________ʽ�ζ��ܡ�
��3���жϴﵽ�ζ��յ��ʵ��������___________________________________��
��4����0.1032mol/L HCl��Һ�ζ�δ֪Ũ�ȵ�NaOH��Һ�����������ʵ������Ӱ�����____________
A.��ʽ�ζ���δ�ñ�������Һ��ϴ
B.��ƿδ�ô���Һ��ϴ
C.�ζ�ǰ�ζ��ܼ�������һ���ݣ��ζ���������ʧ��
D.�ζ�ʱ����Һ������ƿ��
��5��̼��H2CO3��K1=4.3��10-7��K2=5.6��10-11,����H2C2O4��K1=5.9��10-2��K2=6.4��10-50.1 mol/L Na2CO3��Һ��pH____________0.1 mol/L Na2C2O4��Һ��pH��(ѡ��������������С��������������)��������Ũ�ȵIJ�����Һ��̼����Һ�������ϣ���Һ�и�������Ũ�ȴ�С��˳����ȷ����_____________��(ѡ����)
A��c(H+)��c(HC2O4-)��c[HCO3-)��c[CO32-) B��c(HCO3-)��c(HC2O4-)��c(C2O42-)��c(CO32-)
C��c(H+)��c(HC2O4-)��c(C2O42-)��c(CO32-) D��c(H2CO3) ��c(HCO3-)��c(HC2O4-)��c(CO32-)
��6����֪�������ܵ���ʵ��ܶȻ�������Ksp��CaF2����1.5��10��10 ��25��ʱ��2.0��10��3mol��L-1�����ˮ��Һ�У�������ҺpH����������仯�����õ�c��HF����c��F-������ҺpH�ı仯��ϵ������ͼ��ʾ�������������Ϣ�ش��������⣺
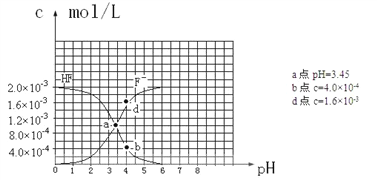
��25��ʱ��HF����ƽ�ⳣ������ֵKa��_______________________________��
��4.0��10��3 mol��L-1HF��Һ��4.0��10��4 mol��L-1 CaCl2��Һ�������ϣ����ڻ��ҺpHΪ4.0�����Ե��ڻ��Һ����ı仯����ͨ����ʽ����˵���Ƿ��г���������_______________________________________
���𰸡� ������Һ �� �������һ�α�Һ����Һ����ɫ�����ɫ�Ұ�����ڲ��ָ� B ���� AC 10-3.45����3.5��10-4�� ��pH=4.0ʱ����Һ����c(F-)=1.6��10-3mol/L����Һ��c(Ca2+)=2.0��l0-4 mol/L��c( Ca2+)��c2(F-)= 5.l��10-10> Ksp( CaF2)���г�������
��������(1)������з�����ӦΪCl2+2KI��2KCl+I2����˿��õ�����Һ��ָʾ����
(2)����Na2S2O3��Һ�ʼ��ԣ�����Ӧ�ü�ʽ�ζ��ܡ�
(3)�ζ�ʱ�����ķ�ӦΪI2+2Na2S2O3=2NaI+ 2Na2S4O6���õ�����Һ��ָʾ������ζ��յ��ʵ�������ǵ������һ�α�Һ����Һ����ɫ����ɫ�����ɫ���ڰ�����ڲ��ָ���
(4)A����ʽ�ζ���δ�ñ�������Һ��ϴ��ʹ����ϡ�ͣ�������Ҫ���������ʹʵ����ƫ�ߣ�B����ƿ������ϴ�������ʹ�ⶨ���ƫ�ߣ�������ƿδ�ô���Һ��ϴ����ʵ������Ӱ�죻C�����ڵζ���������ʧ�����µζ�����Һ���½���ʹ�����������ʵ����ƫ�ߣ�D���ζ�ʱ��С�Ľ���Һ������ƿ�⣬���±�Һ�������ʹʵ����ƫ�ߡ�������ȷ��ΪB��
(5)��֪����ĵ��볣��K1����̼���K1������ĵ��볣��K2Ҳ����̼���K2���������ࡰԽ��Խˮ������ԭ����ͬŨ�ȵ�Na2CO3��Һˮ��̶ȴ���Na2C2O4��Һ�ģ����Na2CO3��Һ��pH����ͬŨ��Na2C2O4��Һ��pH�����ڶ�Ԫ�����Ƿֲ�����ģ����������K1��K2�Ĺ�ϵ����ȷ��A��C����ȷ��B����ȷ��ϵʽΪc(HC2O4-)�� c(C2O42-)�� c(HCO3-)�� c(CO32-)��D����ȷ��ϵʽΪc(H2CO3) ��c(HC2O4-)��c(HCO3-)��c(CO32-)��
(6) ��25��ʱ��HF����ƽ�ⳣ��Ka=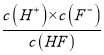 �����Ե�c(F-)=c(HF)ʱ��Ka=c(H+)����ͼ���֪�����еĽ��㴦��Ϊc(F-)=c(HF)������Ӧ��pH=3.45��ΪKa�ĸ���������Ka��10-3.45����3.5��10-4������4.0��10��3 mol��L-1HF��Һ��4.0��10��4 mol��L-1 CaCl2��Һ�������ϵ�˲����Һ��c(HF)=2.0��10-3mol/L����˿���ʹ��ͼ���е����ݽ�����ؼ�������ͼ���֪����pH=4.0ʱ����Һ�е�c(F-)=1.6��10-3mol/L������Ϻ���Һ��c(Ca2+)=2.0��l0-4mol/L������c(Ca2+)��c2(F-)= 2.0��l0-4��(1.6��10-3)2 = 5.l2��10-10 > Ksp(CaF2) ��1.5��10��10�����Ի��г���������
�����Ե�c(F-)=c(HF)ʱ��Ka=c(H+)����ͼ���֪�����еĽ��㴦��Ϊc(F-)=c(HF)������Ӧ��pH=3.45��ΪKa�ĸ���������Ka��10-3.45����3.5��10-4������4.0��10��3 mol��L-1HF��Һ��4.0��10��4 mol��L-1 CaCl2��Һ�������ϵ�˲����Һ��c(HF)=2.0��10-3mol/L����˿���ʹ��ͼ���е����ݽ�����ؼ�������ͼ���֪����pH=4.0ʱ����Һ�е�c(F-)=1.6��10-3mol/L������Ϻ���Һ��c(Ca2+)=2.0��l0-4mol/L������c(Ca2+)��c2(F-)= 2.0��l0-4��(1.6��10-3)2 = 5.l2��10-10 > Ksp(CaF2) ��1.5��10��10�����Ի��г���������


 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ҵ�����Ҫ���л�����ԭ�ϣ�������ϩˮ�Ϸ���������Ӧ�Ļ�ѧ����ʽ���£�C2H4(g) + H2O(g) ![]() C2H5OH(g) ����ͼΪ��ϩ��ƽ��ת�������¶ȣ�T����ѹǿ��P���Ĺ�ϵ[��ʼn(C2H4) : n(H2O) =1:1]��
C2H5OH(g) ����ͼΪ��ϩ��ƽ��ת�������¶ȣ�T����ѹǿ��P���Ĺ�ϵ[��ʼn(C2H4) : n(H2O) =1:1]��

�����й�������ȷ����
A. Y��Ӧ���Ҵ������ʵ�������Ϊ![]()
B. X��Y��Z��Ӧ�ķ�Ӧ��������(X) >��(Y) >��(Z)
C. X��Y��Z��Ӧ��ƽ�ⳣ����ֵ��KX < KY <KZ
D. ����ѹǿ�������¶Ⱦ��������ϩ��ƽ��ת����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ���ǣ� ��
A. ��ԭ�ӹ��ɵľ��岻һ����ԭ�Ӿ��� B. ���Ӿ����еķ����ڲ����й��ۼ�
C. ���Ӿ�����һ���зǼ��Թ��ۼ� D. ���Ӿ����з���һ�����ܶѻ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼���裨SiC����һ�־���������ƽ��ʯ�Ľṹ������̼ԭ�Ӻ�ԭ�ӵ�λ���ǽ���ġ����������־���ٽ��ʯ �ھ���� ��̼���������ǵ��۵㣬�Ӹߵ��͵�˳���ǣ� ��
A. �٢ۢ� B. �ڢۢ� C. �ۢ٢� D. �ڢ٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧ����Ҫ���ռ��������0.5 mol��L-1��NaOH��Һ490mL��ʵ�����ṩ�������������ձ� ��100 mL��Ͳ ��ҩ�� �ܲ����� ��������ƽ�������룩����ش��������⣺
��1������������Ҫ��ȡNaOH���������Ϊ____________��
��2������ʱ������ʹ�õ�������____________������ţ�����ȱ�ٵ������� _______________��______________�������������ƣ�
��3������ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ������ֻ��һ�Σ�__________��
A��������ˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
B����ʢ��NaOH������ձ��м�������ˮ�ܽ�
C�����ձ�������ȴ����Һ�ز�����ע������ƿ��
D��������ƿ�ǽ����������µߵ���ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
��4��ʵ�������õ��������������÷ֱ��ǣ�������________��������________��
��5��������������������н�����������ҺŨ��ƫ�ߵ���_______________��
������ƿʵ��ǰ������ˮϴ�ɾ�����δ���
�ڶ��ݹ۲�Һ��ʱ����
�����ƹ�������©�ˣ�3���в���A
�ܼ�����ˮʱ���������˿̶�
��6����ʵ������г��֣�5���Т���������㽫��δ�����______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʣ��ٰ��ס��ڽ��ʯ����ʯī���ܶ�������ݼ��顡�����Ȼ�̼����笠����ӣ�����ӽṹ����ṹ��Ԫ�д��������������(����)
A. �٢ڢܢݢޢ� B. �٢ڢݢ� C. �ڢ� D. �ۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����С�����������ʵ��ⶨCu��Ag�Ͻ���Ũ����ķ�Ӧ����ʵ��װ����ͼ��

��ش��������⣺
��1��F������������_______________��������____________________________��
��2��ʵ������NH4Cl����ͱ���NaNO2��Һ�ڼ����������Ʊ�N2���÷�Ӧ�Ļ�ѧ����ʽΪ____________________________��װ��A��ʢ�ŵ������Ը��������Һ��������___________________________________________��
��3������ʵ�顣���װ�������Ժ�,��װ����������Ӧ���Լ�,Ȼ��__________����������������ٽ�Bװ�÷�Һ©���е�Ũ���Ỻ���μӵ�������ƿ�С�
��4���ⶨ��������ʵ�������Ӧ����������װ��D������500mL��Һ��ÿ��ȡ��25.00mL��Һ������2��ָʾ������0.05 mol��L-1��NaOH��Һ�ζ������εζ������������±���
�ζ�ǰ���/mL | �ζ������/mL | |
��һ�� | 0.33 | 20.32 |
�ʶ��� | 1.25 | 23.26 |
��=.�� | 1.47 | 21.48 |
װ��D�����ɵ�����Ϊ________mol����Cu-Ag�Ͻ���Ũ���ᷴӦ���������ɵ�NO2�ڱ�״���µ����Ϊ__________mL��
��5���ⶨNO��������ڲⶨNO�����ʱ����E����������ˮ��Һ��ȸ���ܵ�Һ��ߣ�ֱ�Ӷ�����ʹ�ⶨ���������_________���ƫ��ƫС��������ʱӦ������Ͳ��λ��_______������ơ������ơ������Ա�֤����Ͳ�е�Һ���������е�Һ���ƽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ĿǰӦ�����Ľ��������ĵ��ʼ��仯������;�dz��㷺��
��1��K4[Fe(CN)6]������ʳ�εĿ��������̬��ԭ�ӵĵ����Ų�ʽΪ_______________��
��2��Na2[Fe(CN)5(NO)] ���������Ƹ�Ѫѹ��֢��
��Na��N��O�ĵ�һ��������С�����˳��Ϊ_______________��
��CN-��̼ԭ�ӵ��ӻ�������_______________��
��3������������FeCl3�������·ֽ�����ˮ��������
��1molH2O2����������ĿΪ_______________��
��H2O�ķе��H2S�ߵ�ԭ����_______________��
��4������ý����Ҫ�Ĵ�����CO��������ý���õ�����ʧȥ�����ԣ�Fe+5CO=Fe(CO)5����ȥCO�Ļ�ѧ����ʽΪ[Cu(NH3)2]OOCCH3+CO+NH3=[Cu(NH3)3(CO)]OOCCH3��
����CO��Ϊ�ȵ�����ķ���Ϊ_____________�������[Cu(NH3)2]OOCCH3�в����ڵĵ���������_______������ĸ����
a.���Ӽ� b.������ c.��λ�� d.���Թ��ۼ�
��Fe(CO)5�ڿ�����ȼ�յĻ�ѧ����ʽΪ4Fe(CO)5+13O2 ![]() 2Fe2O3+20CO2��Fe2O3�ľ���������___________��
2Fe2O3+20CO2��Fe2O3�ľ���������___________��
�����ľ�����ͼ��ʾ�����þ�����ܶ���ag��cm-3�������������Feԭ�Ӽ�ľ���Ϊ_____cm����NAΪ����٤��������ֵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ����(����)
A. ���ʯ�����е���С̼����6��̼ԭ�ӹ���
B. Na2O2����������������������Ŀ֮��Ϊ1��1
C. 1 mol SiO2�����к�2 mol Si��O��
D. ���ʯ��ѧ�����ȶ����ڸ�����Ҳ�����O2��Ӧ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com