
һ���¶��£���һ���̶��ݻ����ܱ��������N2��g��+3H2(g)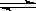 2NH3(g) ��Ӧ���˷�Ӧ�ﵽ��ѧƽ��״̬�ı�־�ǣ���N2��H2��NH3�������������ٸı䣬�������ڵ�ѹǿ����ʱ��ı仯���仯����c(N2)��c(H2)��c(NH3)��1��3��2 ���ܵ�λʱ����ÿ����1molN2��ͬʱ����3molH2����һ��ʱ����N2��H2��NH3��ƽ����ѧ��Ӧ����֮��Ϊ1:3:2 ����������������ܶȲ���
2NH3(g) ��Ӧ���˷�Ӧ�ﵽ��ѧƽ��״̬�ı�־�ǣ���N2��H2��NH3�������������ٸı䣬�������ڵ�ѹǿ����ʱ��ı仯���仯����c(N2)��c(H2)��c(NH3)��1��3��2 ���ܵ�λʱ����ÿ����1molN2��ͬʱ����3molH2����һ��ʱ����N2��H2��NH3��ƽ����ѧ��Ӧ����֮��Ϊ1:3:2 ����������������ܶȲ���
| A���٢ڢۢܢݢ� | B���٢ڢݢ� | C���٢ڢ� | D��ֻ�Т٢� |
 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ����׳��Ƥ�顤����ͬ��ϵ�С������ɽ��������߶���ѧ���ϣ� ���ͣ�021
һ���¶��£���һ���ݻ��̶�������ܱ������н���X(��)��3Y(��)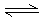 4Z(��)��W(��)��Ӧ���ﵽƽ��״̬�ı�־��
4Z(��)��W(��)��Ӧ���ﵽƽ��״̬�ı�־��
[����]
A����λʱ��������a mol X��ͬʱ����3a mol Y
B����λʱ��������3a mol Y��ͬʱ����4a mol Z
C��������ܶȲ��ٱ仯
D�������ѹǿ���ٱ仯
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com