
��1��A��B��C��D�����ڱ���ǰ10��Ԫ�أ����ǵ�ԭ�Ӱ뾶���μ�С��D�ֱܷ���A��B��C�γɵ���������ȵĶ�ԭ�ӷ���M��N��W������M��N��W�����У�A��B��Cԭ�Ӷ���ȡsp3�ӻ���
��A��B��C�ĵ�һ��������С�����˳��Ϊ_____ (��Ԫ�ط��ű�ʾ)��A22-��C22+��Ϊ�ȵ����壬C22+�ĵ���ʽ__________
��N�ķе����ͬ�������⻯��е�ߵ���Ҫԭ����_____��W���ӵĿռ乹�͵�������_________
��2��E��F��G��Ԫ�ص�ԭ������������������ԭ�ӵ����������Ų���Ϊ4s1��
��FԪ�ػ�̬ԭ�ӵ����Ų�ʽΪ_____
��EԪ�ص��ʵľ���ѻ�ģ��Ϊ_____(����ĸ��
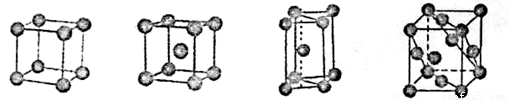
a���������ѻ� b�����������ѻ� c���������ܶѻ� d�������������ܶѻ�
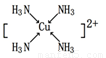
����G����������Һ��ͨ�����N���壬������[G(N)4]2+�����ǿռ乹�ͣ�[G(N)4]2+
�Ľṹ����ʾ��ͼ��ʾΪ_____ (��Ԫ�ط��ű�ʾ)��
��1����C<O<N 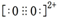
�ڰ����Ӽ��γ������ʹ�۷е����� V��
��2����1s22s22p63s23p63d54s1 �� [Ar]3d54s1
��b
��
��������
�����������1��������������Ԫ����������֪DΪH��AΪC��BΪN��CΪO��MΪCH4��NΪNH3��WΪH2O��
��ͬ����Ԫ�ص�һ������������������������ƣ�����������Ԫ����p�����������һ�����ܴ��ڵ�������Ԫ�ء�����C22-���ӿ�д��O22+���ӵĵ���ʽ��
��NH3�ķе������Ϊ���Ӽ��γ��������ˮ���ӵĿռ乹����V�͡�
��2�����������Ų���4s1��Ԫ����K��Cr��Cu����EΪK��FΪCr��GΪCu��
�ٻ�̬Fԭ�ӵĺ�������Ų�Ϊ1s22s22p63s23p63d54s1��
�ڽ���Na����ľ���Ϊ���������ѻ���
��Cu2+���ṩ�չ����NH3���ṩ�µ��Ӷԣ����߿��γ���λ����
���㣺����Ԫ�غ�������Ų����������ӿռ乹�ͣ�����ԭ���ӻ���ʽ����һ�����ܣ�����������ѻ���ʽ����λ���ı�ʾ�����ݡ�


 ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д� ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪��2NO2��g��?N2O4��g����H��O���ں��º��������£���һ����NO2��N2O4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��t�ı仯��ϵ��ͼ��ʾ��
��֪��2NO2��g��?N2O4��g����H��O���ں��º��������£���һ����NO2��N2O4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��t�ı仯��ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��F������Aԭ�Ӻ���������δ�ɶԵ��ӣ�������B2E�ľ���Ϊ���Ӿ��壬Eԭ�Ӻ���M����ֻ�����ԳɶԵ��ӣ�CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ�D���ʵľ���������ͬ���ڵĵ�����û����ͬ�ģ�Fԭ�Ӻ���������������B��ͬ�����������Ӿ������������������Ϣ���ش��������⣺������ʱ��A��B��C��D��E��F������Ӧ��Ԫ�ط��ű�ʾ��
��֪A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��F������Aԭ�Ӻ���������δ�ɶԵ��ӣ�������B2E�ľ���Ϊ���Ӿ��壬Eԭ�Ӻ���M����ֻ�����ԳɶԵ��ӣ�CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ�D���ʵľ���������ͬ���ڵĵ�����û����ͬ�ģ�Fԭ�Ӻ���������������B��ͬ�����������Ӿ������������������Ϣ���ش��������⣺������ʱ��A��B��C��D��E��F������Ӧ��Ԫ�ط��ű�ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com