
| A�� | �ؽ�������Ag+��Cu2+��K+���ɵ��µ����ʱ��� | |
| B�� | S02��NxOy�����ڷǽ��������Ҳ�������������� | |
| C�� | ���ֿ�����ɢ�ڿ����п��ܻ��γɶ�������� | |
| D�� | ���ͱ��������ﶼ�Ƿ����� |
���� A��K+�����ؽ������ӣ�
B��NxOy���δ֪����һ�������������
C��������ж����ЧӦ��
D��������ͨ��ָ�����к��б����ṹ��̼�⻯���
��� �⣺A��K+�����ؽ������ӣ����ɵ��µ����ʱ��ԣ���A����
B����������Ϊ�����������NxOy���δ֪����һ���������������B����
C�������ɢ������ֱ����СΪ1-100���ף�ֱ����1-100���Ŀ������ɢ�ڿ����п��γɽ��壬��C��ȷ��
D��������������ܺ���������һ����������D����
��ѡC��
���� ���⿼���˵����ʡ���������ʡ�������������������Ķ��壬�漰֪ʶ��㣬��Ϊ������֪ʶ�������������ʵ����ʺ����ǽ���Ĺؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ��ѧ��Ӧ�������ӷ���ʽ | ���� |
| A | Fe3O4�����ϡ���ᷴӦ��Fe3O4+8H+��2Fe3++Fe2++4H2O | ��ȷ |
| B | �ڸ���NH4Fe��SO4��2��Һ����μ���Ba��OH��2��Һ�� 2Fe3++3SO42-+3Ba2++6OH-��3BaSO4��+2Fe��OH��3�� | ��ȷ |
| C | ��ϡ��ˮ��ͨ�����CO2��NH3•H2O+CO2��NH4++HCO3- | ��ȷ |
| D | FeBr2��Һ������ʵ�����Cl2��Ӧ�� 2Fe2++2Br-+2Cl2��2Fe3++4Cl-+Br2 | ����Fe2+��Br-�Ļ�ѧ������֮��ӦΪ1��2 |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
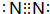 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
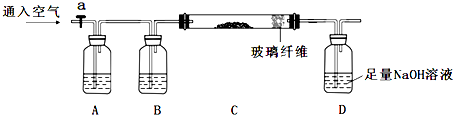
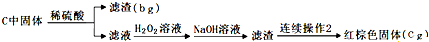
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ������ | B�� | ������ | C�� | �˵���� | D�� | �ܲ���ܼ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʱ�䣨min�� Ũ�ȣ�mol/L�� | 0 | 10 | 20 | 30 | 40 | 50 |
| NO | 1.00 | 0.58 | 0.40 | 0.40 | 0.48 | 0.48 |
| N2 | 0 | 0.21 | 0.30 | 0.30 | 0.36 | 0.36 |
| CO2 | 0 | 0.21 | 0.30 | 0.30 | 0.36 | 0.36 |
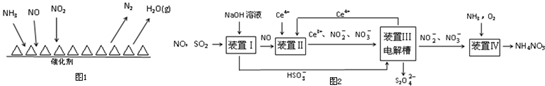
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ̼ | B�� | �� | C�� | �� | D�� | �� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com