
A��B��C��D�����ֶ�����Ԫ�أ�E�ǹ���Ԫ�أ�A��B��Cͬ���ڣ�C��Dͬ���壬A��ԭ�ӽṹʾ��ͼΪ��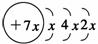 ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
(1)д������Ԫ�صķ��ţ�A________��B________��C________��D________��
(2)�û�ѧʽ��ʾ��������Ԫ��������������Ӧˮ����������ǿ����________��������ǿ����________��
(3)��Ԫ�ط��ű�ʾD�������ڵ�һ����������Ԫ����________���縺������Ԫ����________��
(4)����D�ĺ�������Ų�ͼ________�������Ų���ѭ��________ԭ����________����
��������A��ԭ�ӽṹʾ��ͼ��֪��x��2��ԭ������Ϊ14��A�ǹ�Ԫ�أ���B����Ԫ�أ�C���������ӵ��Ų�ʽΪ3s23p3������Ԫ�أ��������DԪ���ǵ�Ԫ�أ�E�ĵ����Ų�ʽΪ[Ar]3d64s2���˵����Ϊ18��8��26������Ԫ�أ�λ��d���ĵ������ڡ����塣�ǽ�������ǿ����NԪ�أ���Ӧ��HNO3������ǿ����������ǿ����NaԪ�أ���Ӧ��NaOH������ǿ��D�������ڵ�һ����������Ԫ����ϡ�������ʣ��縺������Ԫ���Ƿǽ�����ǿ�ķ���
�𰸡�(1)Si��Na��P��N��(2)HNO3��NaOH



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�¶��£���pH��6������ˮ�м���NaHSO4���壬�����¶Ȳ��䣬�����Һ��pHΪ2�����жԸ���Һ�������У�����ȷ����(����)
A�����¶ȸ���25��
B����ˮ�������[H��]��1.0��10��10 mol��L��1
C������NaHSO4����������ˮ�ĵ���
D�����¶��¼�������pH��12��NaOH��Һ��ʹ����Һǡ�ó�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ܵ������ˮ��Һ�д����ŵ���ƽ�⡣�ڳ����£���Һ�������Ũ�������ǻ�ѧ������Ϊ���εij˻���һ�����������ܶȻ�����(Ksp)�����磺Cu(OH)2(s)
 Cu2��(aq)��2OH����Ksp��[Cu2��]��[OH��]2��2��10��20 mol3��L��3������Һ�и�����Ũ�ȷ��εij˻������ܶȻ�ʱ���������������֮�����ܽ⡣
Cu2��(aq)��2OH����Ksp��[Cu2��]��[OH��]2��2��10��20 mol3��L��3������Һ�и�����Ũ�ȷ��εij˻������ܶȻ�ʱ���������������֮�����ܽ⡣
(1)ijCuSO4��Һ��[Cu2��]��0.02 mol��L��1����Ҫ����Cu(OH)2������Ӧ������Һ��pH��ʹ֮����________��
(2)Ҫʹ0.2 mol��L��1 CuSO4��Һ��Cu2��������Ϊ��ȫ(ʹCu2��Ũ�Ƚ���ԭ����ǧ��֮һ)����Ӧ����Һ�����NaOH��Һ��ʹ��ҺpHΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Կ��淴ӦaA(g)��bB(g)
 cC(g)��dD(g)���ﵽƽ��ʱ�������ʵ����ʵ���Ũ��Ӧ�������¹�ϵ��
cC(g)��dD(g)���ﵽƽ��ʱ�������ʵ����ʵ���Ũ��Ӧ�������¹�ϵ��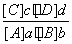 ��K��KΪһ��������Ϊ��ѧƽ�ⳣ�����䷴Ӧ��Kֵֻ���¶��йأ����з�Ӧ��CO(g)��H2O(g)
��K��KΪһ��������Ϊ��ѧƽ�ⳣ�����䷴Ӧ��Kֵֻ���¶��йأ����з�Ӧ��CO(g)��H2O(g)
 CO2(g)��H2(g)����H<0����850��ʱ��K��1��
CO2(g)��H2(g)����H<0����850��ʱ��K��1��
(1)�������¶ȵ�950�棬�ﵽƽ��K________(����ڡ�����С�ڡ����ڡ�)1��
(2)850��ʱ������һ�ݻ��ɱ���ܱ�������ͬʱ����1.0 mol CO,3.0 mol H2O,1.0 mol CO2��x mol H2����
�ٵ�x��5.0ʱ������ƽ����________(�����Ӧ�����淴Ӧ��)�����ƶ���
����Ҫʹ������Ӧ��ʼʱ������Ӧ������У���xӦ�����������__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������Ԫ�صĻ�̬ԭ�ӵĵ����Ų�ʽ���£�
��1s22s2 2p63s23p4����1s22s22p63s23p3��
��1s22s2 2p3����1s22s22p5��
�������йرȽ�����ȷ���� (����)��
A����һ�����ܣ���>��>��>��
B��ԭ�Ӱ뾶����>��>��>��
C���縺�ԣ���>��>��>��
D����������ϼۣ���>�ۣ���>��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ݱ�����������M�Է��ѻ�ù���нϺõ��־����ԣ���ϳ�·������ͼ��ʾ��

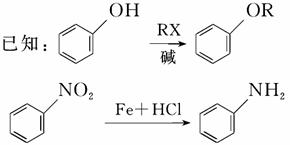
���������գ�
(1)д����Ӧ���ͣ�
��Ӧ��________����Ӧ��________��
(2)д���ṹ��ʽ��
A_____________________________________________________________��
E__________________________________________________________��
(3)д����Ӧ�ڵĻ�ѧ����ʽ��_______________________________________
_________________________________________________________________��
(4)B�ĺ������ṹ��ͬ���칹���У���һ���ܷ�������ˮ�⣬д����������ͬ���칹���еĹ�����(���ǻ�����)���Լ������ֵ�����
�Լ�(��̪����)��___________________________________________��
����_____________________________________________________��
(5)д������C�ĺ������ṹ��ֻ��4�ֲ�ͬ��ѧ������ԭ�ӵ�ͬ���칹��Ľṹ��ʽ��
_________________________________________________________
_________________________________________________________��
(6)��Ӧ�١���Ӧ�ڵ��Ⱥ�����ܵߵ��������ԭ��_________________
____________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
PbO2�Ǻ�ɫ���壬���ȷֽ�ΪPb�ģ�4�ͣ�2�۵Ļ���������4�۵�Pb������Ũ��������Cl2���ֽ�1 mol PbO2���ȷֽ�õ�O2����ʣ������м���������Ũ����õ�Cl2��O2��Cl2�����ʵ���֮��Ϊ3��2����ʣ��������ɼ����ʵ���֮���� (����)��
A��1��1��ϵ�Pb3O4��PbO
B��1��2��ϵ�PbO2��Pb3O4
C��1��4��1��ϵ�PbO2��Pb3O4��PbO
D��1��4��1��ϵ�PbO2��Pb3O4��PbO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ð�Һ������ӡˢп��ʱ����ϡ���ḯʴп���õ��ġ���Һ��(����������Cl����Fe3��)��ij��ѧ��ȤС�����á��ð�Һ����ȡZn(NO3)2��6H2O�Ĺ������£�

��֪��Zn(NO3)2��6H2O��һ����ɫ���壬ˮ��Һ�����ԣ�Zn(NO3)2����Ӧ���õ��IJ���������ԡ�
(1)���ð�Һ�������ʵ���Ҫ�ɷ���______(�ѧʽ����ͬ)��
(2)�ڲ������б���pH��8��Ŀ����
________________________________________________________________________��
(3)���������Ҫ�ɷ���____________��
(4)�������м�����е�Ŀ����________________���˲�����������������
________________________________________________________________________��
(5)�����ܱ���pH��2��Ŀ����____________���˲����������õ���Ҫ������
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�ʵ����ȷ����
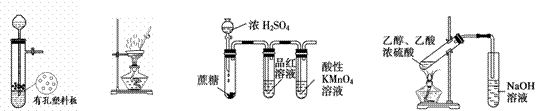 |
ͼ1 ͼ2 ͼ3 ͼ4
A.ͼ1װ������Cu��ŨH2SO4��Ӧ��ȡ������SO2����
B.ͼ2װ����������Al(OH)3
C.ͼ3װ�����ڼ���Ũ���������Ƿ�Ӧ�����Ķ�������
D.ͼ4װ������ʵ�����Ʊ���������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com