
ȡA��B�������ʵ���Ũ����ȵ�NaOH��Һ�������Ϊ50mL���ֱ�������ͨ��һ������CO2 ���ٷֱ�ϡ��Ϊ100mL��
��1����NaOH��Һ��ͨ��һ������CO2����Һ�е����ʵ���ɿ����ǣ�
��2����ϡ�ͺ����Һ�зֱ���μ���0.1mol��L��1 �����ᣬ������CO2���������״��������������������ϵ����ͼ��ʾ��
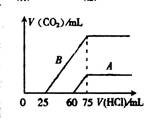
�� �ֱ�����������������Һ�е������� ��
ԭNaOH��Һ�����ʵ���Ũ���� ��
�� A���߱�����NaOH��Һͨ��CO2��������Һ�е�����
�� �������ᷴӦ����CO2���������� mL(��״��)��
�� B���߱�����ԭNaOH��Һͨ��CO2���������ʵĻ�ѧʽΪ �� �����ʵ���֮��Ϊ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��Ҫ����д���пհף�
��Ҫ����д���пհף��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ȡA��B�������ʵ���Ũ����ȵ�NaOH��Һ�������Ϊ50mL���ֱ�������ͨ��һ������CO2 ���ٷֱ�ϡ��Ϊ100mL��
��1����NaOH��Һ��ͨ��һ������CO2����Һ�е����ʵ���ɿ����ǣ�
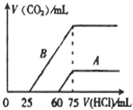
��2����ϡ�ͺ����Һ�зֱ���μ���0.1mol��L��1 �����ᣬ������CO2���������״��������������������ϵ����ͼ��ʾ��
�� �ֱ�����������������Һ�е������� ��
ԭNaOH��Һ�����ʵ���Ũ���� ��
�� A���߱�����NaOH��Һͨ��CO2��������Һ�е�����
�� �������ᷴӦ����CO2���������� mL(��״��)��
�� B���߱�����ԭNaOH��Һͨ��CO2���������ʵĻ�ѧʽΪ �� �����ʵ���֮��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�������ʡ��һ�и߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ��ʴ���
ȡA��B�������ʵ���Ũ����ȵ�NaOH��Һ�������Ϊ50 mL���ֱ�������ͨ��һ������CO2���ٷֱ�ϡ��Ϊ100 mL��
(1)��NaOH��Һ��ͨ��һ������CO2����Һ�����ʵ���ɿ����ǣ�
��________����________����________����________��
(2)��ϡ�ͺ����Һ�зֱ���μ�0.1 mol/L�����ᣬ������CO2�����(��״��)
����������������ϵ��ͼ��ʾ��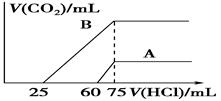
�ٷֱ������������������Һ�е�������________��ԭNaOH��Һ�����ʵ���Ũ��Ϊ________��
��A���߱�����ԭ��Һͨ��CO2������������HCl��Ӧ����CO2����������________mL(��״��)��
��B���߱�����ԭ��Һͨ��CO2���������ʵĻ�ѧʽΪ________�������ʵ���֮��Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012����������һ�и����ڶ����¿���ѧ�Ծ� ���ͣ������
ȡA��B�������ʵ���Ũ����ȵ�NaOH��Һ�������Ϊ50mL���ֱ�������ͨ��һ������CO2 ���ٷֱ�ϡ��Ϊ100mL��
��1����NaOH��Һ��ͨ��һ������CO2����Һ�е����ʵ���ɿ����ǣ�
��2����ϡ�ͺ����Һ�зֱ���μ���0.1mol��L��1 �����ᣬ������CO2���������״��������������������ϵ����ͼ��ʾ��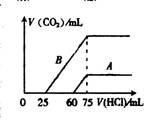
�� �ֱ�����������������Һ�е������� ��
ԭNaOH��Һ�����ʵ���Ũ���� ��
�� A���߱�����NaOH��Һͨ��CO2��������Һ�е�����
�� �������ᷴӦ����CO2���������� mL(��״��)��
�� B���߱�����ԭNaOH��Һͨ��CO2���������ʵĻ�ѧʽΪ �� �����ʵ���֮��Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com