
 »Ð“∫÷–
»Ð“∫÷–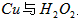 ∑¥”¶…˙≥…
∑¥”¶…˙≥… µƒ»»ªØ—ß∑Ω≥Ã ΩŒ™________________________°£
µƒ»»ªØ—ß∑Ω≥Ã ΩŒ™________________________°£  ∫Õ3£Æ0
∫Õ3£Æ0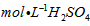 µƒªÏ∫œ»Ð“∫¥¶¿Ì£¨≤‚µ√≤ªÕ¨Œ¬∂»œ¬Õ≠µƒ∆Ωæ˘»ÐΩ‚ÀŸ¬ £®º˚œ¬±Ì£©°£
µƒªÏ∫œ»Ð“∫¥¶¿Ì£¨≤‚µ√≤ªÕ¨Œ¬∂»œ¬Õ≠µƒ∆Ωæ˘»ÐΩ‚ÀŸ¬ £®º˚œ¬±Ì£©°£ 
 µƒ»Ð“∫÷–º”»Î“ª∂®¡øµƒ
µƒ»Ð“∫÷–º”»Î“ª∂®¡øµƒ »Ð“∫£¨º”»»£¨…˙≥…
»Ð“∫£¨º”»»£¨…˙≥… ≥¡µÌ°£÷∆±∏
≥¡µÌ°£÷∆±∏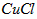

| ƒÍº∂ | ∏þ÷–øŒ≥à | ƒÍº∂ | ≥ı÷–øŒ≥à |
| ∏þ“ª | ∏þ“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı“ª | ≥ı“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏þ∂˛ | ∏þ∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı∂˛ | ≥ı∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏þ»˝ | ∏þ»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı»˝ | ≥ı»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° |
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
| 1 |
| 2 |
| Œ¬∂»£®°Ê£© | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| Õ≠∆Ωæ˘»ÐΩ‚ÀŸ¬ £®°¡10-3mol?L-1?min-1£© |
7.34 | 8.01 | 9.25 | 7.98 | 7.24 | 6.73 | 5.76 |
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
| Œ¬∂»£®°Ê£© | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| Õ≠∆Ωæ˘»ÐΩ‚ÀŸ¬ £®°¡10-3 mol?L-1?min-1£© | 7.34 | 8.01 | 9.25 | 7.98 | 7.24 | 6.73 | 5.76 |
| ||
| ||
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
(1)œ¬¡–¥¶¿Ì”°À¢µÁ¬∑∞Â∑«Ω Ù∑€ƒ©µƒ∑Ω∑®÷–£¨≤ª∑˚∫œª∑æ≥±£ª§¿ÌƒÓµƒ «_______(ÃÓ◊÷ƒ∏)°£
A.»»¡—Ω‚–Œ≥…»º”Õ
B.¬∂ÃÏ∑Ÿ…’
C.◊˜Œ™”–ª˙∏¥∫œΩ®÷˛≤ƒ¡œµƒ‘≠¡œ
D.÷±Ω”ÃÓ¬Ò
(2)”√H2O2∫ÕH2SO4µƒªÏ∫œ»Ð“∫ø…»Ð≥ˆ”°À¢µÁ¬∑∞ÂΩ Ù∑€ƒ©÷–µƒÕ≠°£“—÷™£∫
Cu(s)+2H+ (aq)====Cu2+ (aq)+H2(g) ¶§H£Ω64.39 kJ°§mol-1
2H2O2(l)====2H2O(l)+O2(g) ¶§H£Ω-196.46 kJ°§mol-1
![]() ====H2O(l) ¶§H£Ω-285.84 kJ°§mol-1
====H2O(l) ¶§H£Ω-285.84 kJ°§mol-1
‘⁄H2SO4»Ð“∫÷–Cu”ÎH2O2∑¥”¶…˙≥…Cu2+∫ÕH2Oµƒ»»ªØ—ß∑Ω≥Ã ΩŒ™_____________________°£
(3)øÿ÷∆∆‰À˚Ãıº˛œýÕ¨£¨”°À¢µÁ¬∑∞µƒΩ Ù∑€ƒ©”√10%H2O2∫Õ3.0 mol°§L-1 H2SO4µƒªÏ∫œ»Ð“∫¥¶¿Ì£¨≤‚µ√≤ªÕ¨Œ¬∂»œ¬Õ≠µƒ∆Ωæ˘»ÐΩ‚ÀŸ¬ (º˚œ¬±Ì)°£
Œ¬∂»/°Ê | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
Õ≠∆Ωæ˘»ÐΩ‚ÀŸ¬ /°¡10-3 mol°§L-1°§min-1 | 7.34 | 8.01 | 9.25 | 7.98 | 7.24 | 6.73 | 5.76 |
µ±Œ¬∂»∏þ”⁄
(4)‘⁄÷¥ø∫ÛµƒCuSO4»Ð“∫÷–º”»Î“ª∂®¡øµƒNa2SO3∫ÕNaCl»Ð“∫£¨º”»»£¨…˙≥…CuCl≥¡µÌ°£÷∆±∏CuClµƒ¿Î◊”∑Ω≥Ã Ω «_________________________________________________________°£
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫2015ΩÏ∞≤ª’ °∏þ∂˛…œ—ß∆⁄∆⁄÷–øº ‘ªØ—ß ‘æÌ£®Ω‚Œˆ∞Ê£© –գ∫ÃÓø’Â
∑œæ…”°À¢µÁ¬∑∞µƒªÿ ’¿˚”√ø… µœ÷◊ ‘¥‘Ÿ…˙£¨≤¢ºı…ŸŒ€»æ°£∑œæ…”°À¢µÁ¬∑∞Âæ≠∑€ÀÈ∑÷¿Î£¨ƒÐµ√µΩ∑«Ω Ù∑€ƒ©∫ÕΩ Ù∑€ƒ©°£
£®1£©œ¬¡–¥¶¿Ì”°À¢µÁ¬∑∞Â∑«Ω Ù∑€ƒ©µƒ∑Ω∑®÷–£¨≤ª∑˚∫œª∑æ≥±£ª§¿ÌƒÓµƒ « £®ÃÓ◊÷ƒ∏£©°£
A£Æ»»¡—Ω‚–Œ≥…»º”ÕB£Æ¬∂ÃÏ∑Ÿ…’ C£Æ◊˜Œ™”–ª˙∏¥∫œΩ®÷˛≤ƒ¡œµƒ‘≠¡œD£Æ÷±Ω”ÃÓ¬Ò
£®2£©”√H2O2∫ÕH2SO4µƒªÏ∫œ»Ð“∫ø…»Ð≥ˆ”°À¢µÁ¬∑∞ÂΩ Ù∑€ƒ©÷–µƒÕ≠°£“—÷™£∫
Cu(s)£´2H£´(aq)=Cu2£´(aq)£´H2(g) °˜H=64.39kJ°§mol£≠1
2H2O2(l)=2H2O(l)£´O2(g) °˜H=£≠196.46kJ°§mol£≠1
H2(g)£´1/2O2(g)=H2O(l)
°˜H=£≠285.84kJ°§mol£≠1
‘⁄ H2SO4»Ð“∫÷–Cu”ÎH2O2∑¥”¶…˙≥…Cu2£´∫ÕH2Oµƒ»»ªØ—ß∑Ω≥Ã ΩŒ™ °£
£®3£©øÿ÷∆∆‰À˚Ãıº˛œýÕ¨£¨”°À¢µÁ¬∑∞µƒΩ Ù∑€ƒ©”√10®GH2O2∫Õ3.0mol°§L£≠1H2SO4µƒªÏ∫œ»Ð“∫¥¶¿Ì£¨≤‚µ√≤ªÕ¨Œ¬∂»œ¬Õ≠µƒ∆Ωæ˘»ÐΩ‚ÀŸ¬ £®º˚œ¬±Ì£©°£
|
Œ¬∂»£®°Ê£© |
20 |
30 |
40 |
50 |
60 |
70 |
80 |
|
Õ≠∆Ωæ˘»ÐΩ‚ÀŸ¬ £®°¡10-3 mol°§L-1°§min-1£© |
7.34 |
8.01 |
9.25 |
7.98 |
7.24 |
6.73 |
5.76 |
µ±Œ¬∂»∏þ”⁄40°Ê ±£¨Õ≠µƒ∆Ωæ˘»ÐΩ‚ÀŸ¬ ÀÊ◊≈∑¥”¶Œ¬∂»…˝∏þ∂¯œ¬Ωµ£¨∆‰÷˜“™‘≠“Ú « °£
£®4£©‘⁄÷¥ø∫ÛµƒCuSO4»Ð“∫÷–º”»Î“ª∂®¡øµƒNa2SO3∫ÕNaCl»Ð“∫£¨º”»»£¨…˙≥…CuCl≥¡µÌ°£÷∆±∏CuClµƒ¿Î◊”∑Ω≥Ã Ω « °£
£®5£© “—÷™œýÕ¨Ãıº˛œ¬£∫
4Ca5(PO4)3F(s)+3SiO2(s)=6Ca3(PO4)2(s)+2CaSiO3(s)+SiF4(g) £ª°˜H1
2Ca3(PO4)2(s)+10C(s)=P4(g)+6CaO(s)+10CO(g)£ª°˜H2
SiO2(s)+CaO(s)=CaSiO3(s) £ª°˜H3
4Ca5(PO4)3F(s)+21SiO2(s)+30C(s)=3P4(g)+20CaSiO3(s)+30CO(g)+SiF4(g) £ª H
H
”√°˜H1°¢°˜H2∫Õ°˜H3±Ì æ H£¨
H£¨ H=
°£
H=
°£
£®6£©“—÷™1 g FeS2(s)ÕÍ»´»º…’…˙≥…∑≈≥ˆ7.1 kJ»»¡ø£¨FeS2»º…’∑¥”¶µƒ»»ªØ—ß∑Ω≥Ã ΩŒ™ °£
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫2014ΩÏ…¬Œ˜ ¶¥Û∏Ω÷–∏þ“ªœ¬—ß∆⁄∆⁄÷–øº ‘ªØ—ß ‘æÌ£®Ω‚Œˆ∞Ê£© –գ∫ÃÓø’Â
£®8∑÷£©∑œæ…”°À¢µÁ¬∑∞µƒªÿ ’¿˚”√ø… µœ÷◊ ‘¥‘Ÿ…˙£¨≤¢ºı…ŸŒ€»æ°£∑œæ…”°À¢µÁ¬∑∞Âæ≠∑€ÀÈ∑÷¿Î£¨ƒÐµ√µΩ∑«Ω Ù∑€ƒ©∫ÕΩ Ù∑€ƒ©°£
£®1£©œ¬¡–¥¶¿Ì”°À¢µÁ¬∑∞Â∑«Ω Ù∑€ƒ©µƒ∑Ω∑®÷–£¨≤ª∑˚∫œª∑æ≥±£ª§¿ÌƒÓµƒ « £®ÃÓ◊÷ƒ∏£©°£
A.»»¡—Ω‚–Œ≥…»º”Õ B.¬∂ÃÏ∑Ÿ…’
C.◊˜Œ™”–ª˙∏¥∫œΩ®÷˛≤ƒ¡œµƒ‘≠¡œ D.÷±Ω”ÃÓ¬Ò
£®2£©”√ µƒªÏ∫œ»Ð“∫ø…»Ð≥ˆ”°À¢µÁ¬∑∞ÂΩ Ù∑€ƒ©÷–µƒÕ≠°£
µƒªÏ∫œ»Ð“∫ø…»Ð≥ˆ”°À¢µÁ¬∑∞ÂΩ Ù∑€ƒ©÷–µƒÕ≠°£ “—÷™£∫
“—÷™£∫
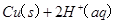 ====
====
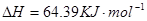
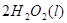 ====
====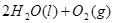

 ====
====


‘⁄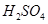 »Ð“∫÷–
»Ð“∫÷– ”Î
”Î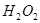 ∑¥”¶…˙≥…
∑¥”¶…˙≥…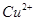 ∫Õ
∫Õ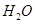 µƒ»»ªØ—ß∑Ω≥Ã ΩŒ™
µƒ»»ªØ—ß∑Ω≥Ã ΩŒ™
°£
£®3£©‘⁄298Kœ¬£¨C°¢Alµƒµ•÷ ∏˜1molÕÍ»´»º…’£¨∑÷±∑≈≥ˆ»»¡øaKJ∫ÕbKJ°£”÷÷™“ª∂®Ãıº˛œ¬£¨AlƒÐΩ´C¥”CO2÷√ªª≥ˆ¿¥£¨–¥≥ˆ¥À÷√ªª∑¥”¶µƒ»»ªØ—ß∑Ω≥Ã Ω °£
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
∞Ÿ∂»÷¬–≈ - ¡∑œ∞≤·¡–±Ì - ‘¡–±Ì
∫˛±± °ª•¡™Õ¯Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®∆Ωî | Õ¯…œ”–∫¶–≈œ¢æŸ±®◊®«¯ | µÁ–≈’©∆≠柱®◊®«¯ | …Ê¿˙ ∑–ÈŒÞ÷˜“”–∫¶–≈œ¢æŸ±®◊®«¯ | …Ê∆Û«÷»®æŸ±®◊®«¯
Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®µÁª∞£∫027-86699610 柱®” œ‰£∫58377363@163.com