
| ·ú»ŻÎï | NaF | MgF2 | SiF4 |
| ÈÛ”ă/K | 1266 | 1534 | 183 |
| | CŁO | C=O | CĄÔO |
| CO | 357Łź7 | 798Łź9 | 1071Łź9 |
| | NŁN | N=N | NĄÔN |
| N2 | 154Łź8 | 418Łź4 | 941Łź7 |

| Äꌶ | žßÖĐżÎłÌ | Äꌶ | łőÖĐżÎłÌ |
| žßÒ» | žßÒ»Ăâ·ŃżÎłÌÍÆŒöŁĄ | łőÒ» | łőÒ»Ăâ·ŃżÎłÌÍÆŒöŁĄ |
| žß¶ț | žß¶țĂâ·ŃżÎłÌÍÆŒöŁĄ | łő¶ț | łő¶țĂâ·ŃżÎłÌÍÆŒöŁĄ |
| žßÈę | žßÈęĂâ·ŃżÎłÌÍÆŒöŁĄ | łőÈę | łőÈęĂâ·ŃżÎłÌÍÆŒöŁĄ |
żÆÄżŁșžßÖĐ»ŻŃ§ ÀŽÔŽŁșČ»Ïê ÌâĐÍŁșÌîżŐÌâ
Č鿎Žđ°žșÍœâÎö>>
żÆÄżŁșžßÖĐ»ŻŃ§ ÀŽÔŽŁșČ»Ïê ÌâĐÍŁș”„ŃĄÌâ

| AŁźąÙąÛąÚ | BŁźąÚąÛąÙ | CŁźąÛąÙąÚ | DŁźąÚąÙąÛ |
Č鿎Žđ°žșÍœâÎö>>
żÆÄżŁșžßÖĐ»ŻŃ§ ÀŽÔŽŁșČ»Ïê ÌâĐÍŁș”„ŃĄÌâ
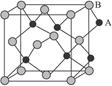
| AŁźAB | BŁźA2B | CŁźAB2 | DŁźA2B3 |
Č鿎Žđ°žșÍœâÎö>>
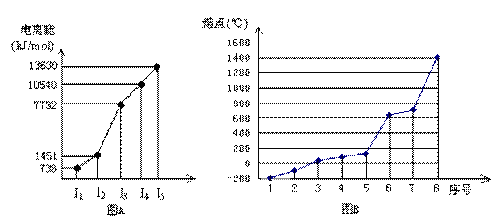
żÆÄżŁșžßÖĐ»ŻŃ§ ÀŽÔŽŁșČ»Ïê ÌâĐÍŁșŒÆËăÌâ
Č鿎Žđ°žșÍœâÎö>>
żÆÄżŁșžßÖĐ»ŻŃ§ ÀŽÔŽŁșČ»Ïê ÌâĐÍŁș”„ŃĄÌâ
| AŁźÁœÖÖÔȘËŰŚéłÉ”Ä·ÖŚÓÖĐÒ»¶šÖ»ÓĐŒ«ĐÔŒü |
| BŁźÔŚÓŸ§ÌćÖĐčČŒÛŒüÔœÇżŁŹÈÛ”ăÔœžß |
| CŁźŸ§ÌćÖĐ·ÖŚÓŒäŚśÓĂÁŠÔœŽóŁŹ·ÖŚÓÔœÎȶš |
| DŁźÓÉŒ«ĐÔčČŒÛŒüœáșÏ”Ä·ÖŚÓÒ»¶šÊÇŒ«ĐÔ·ÖŚÓ |
Č鿎Žđ°žșÍœâÎö>>
żÆÄżŁșžßÖĐ»ŻŃ§ ÀŽÔŽŁșČ»Ïê ÌâĐÍŁș”„ŃĄÌâ
| AŁźO2ĄąSĄąBr2 | BŁźCO2ĄąKClĄąSiO2 | CŁźNaĄąWĄąNaCl | DŁźH2OĄąH2SĄąH2Se |
Č鿎Žđ°žșÍœâÎö>>
żÆÄżŁșžßÖĐ»ŻŃ§ ÀŽÔŽŁșČ»Ïê ÌâĐÍŁș”„ŃĄÌâ
| AŁźœđÊôĂŸ | BŁźÂÈ»ŻÄÆŸ§Ìć | CŁź±ù | DŁźŸ§Ìćčè |
Č鿎Žđ°žșÍœâÎö>>
żÆÄżŁșžßÖĐ»ŻŃ§ ÀŽÔŽŁșČ»Ïê ÌâĐÍŁș”„ŃĄÌâ
| AŁź22.4LCO2Óë22.4L COËùșŹŃőÔŚÓÊęÄżÖź±È |
| BŁźÔÚNa2O2Ÿ§ÌćÖĐŃôÀëŚÓÓëÒőÀëŚÓ”ÄÎïÖÊ”ÄÁżÖź±È |
| CŁźÏà͏ζÈÏÂŁŹ0.2molĄ€L-1ŽŚËáÈÜÒșÓë0.1molĄ€L-1ŽŚËáÖĐ”ÄcŁšH+Ł©Öź±È |
| DŁźÒșĂæŸùÔÚĄ°0Ą±żÌ¶ÈʱŁŹ50mlŒîÊœ”ζščÜșÍ25mlŒîÊœ”ζščÜËùÊąÈÜÒș”ÄÌć»ęÖź±È |
Č鿎Žđ°žșÍœâÎö>>
°Ù¶ÈÖÂĐĆ - Á·Ï°ČáÁбí - ÊÔÌâÁбí
șț±±ÊĄ»„ÁȘÍű΄·šșÍČ»ÁŒĐĆÏąŸÙ±šÆœÌš | ÍűÉÏÓĐșŠĐĆÏąŸÙ±šŚšÇű | ”çĐĆŐ©ÆŸÙ±šŚšÇű | ÉæÀúÊ·ĐéÎȚÖśÒćÓĐșŠĐĆÏąŸÙ±šŚšÇű | ÉæÆóÇÖÈšŸÙ±šŚšÇű
΄·šșÍČ»ÁŒĐĆÏąŸÙ±š”ç»°Łș027-86699610 ŸÙ±šÓÊÏäŁș58377363@163.com