
�Ѱ�(��Ҫ�ɷ���TiO2)���㷺�������ᡢ���ϡ���ֽ����ҵ�����������Ҵ���ˮ������Ĵ�������ͼ����������(��Ҫ�ɷ�FeTiO3����������)Ϊ��Ҫԭ�������Ѱ۲���ø���ƷFeSO4��7H2O�Ĺ�������ͼ��
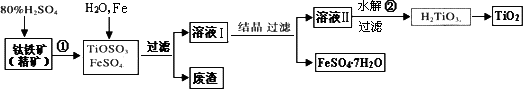
(1)�����������ᷢ����Ӧ�ٵĻ�ѧ����ʽΪ________����TiOSO4��FeSO4��Һ�м���Fe��Ŀ����________��
(2)��Һ����TiOSO4�ڼ��������·���ˮ�ⷴӦ�ڵ����ӷ���ʽΪ________���ɻ������õ�������________��
(3)Ϊ�ⶨ��Һ����TiOSO4�ĺ���������ȡ������Һ10 mL��ˮϡ����100 mL���ӹ������ۣ������ʹ����ȫ��Ӧ��3TiO2+��Al��6H+��3Ti3+��Al3+��3H2O�����˺�ȡ����Һ20.00 mL�������еμ�2��3��KSCN��Һ��ָʾ������________(��һ�ֲ�������������)�μ�0.1000 mol��L��1��FeCl3��Һ������Һ���ֺ�ɫ�ﵽ�ζ��յ㣬��ȥ��30.00 mL��FeC13��Һ��������Һ��TiOSO4�����ʵ���Ũ����________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| TiCl4 | SiCl4 | |
| �۵�/�� | -25.0 | -68.8 |
| �е�/�� | 136.4 | 57.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ��ͨ��ͨ���������ص��ȵ�ר���⻯ѧ�Ծ� ���ͣ������
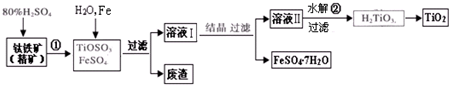
(12��) �Ѱۣ���Ҫ�ɷ���TiO2�����㷺�������ᡢ���ϡ���ֽ����ҵ�����������Ҵ���ˮ������Ĵ�������ͼ������������Ҫ�ɷ�FeTiO3������������Ϊ��Ҫԭ�������Ѱ۲���ø���ƷFeSO4��7H2O�Ĺ�������ͼ��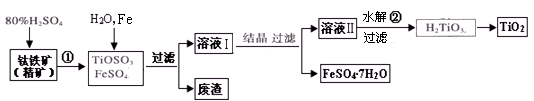
(1)�����������ᷢ����Ӧ�ٵĻ�ѧ����ʽΪ ����TiOSO4��FeSO4��Һ�м���Fe��Ŀ���� ��
(2)��Һ����TiOSO4�ڼ��������·���ˮ�ⷴӦ�ڵ����ӷ���ʽΪ ���ɻ������õ������� ��
(3)Ϊ�ⶨ��Һ����TiOSO4�ĺ���������ȡ������Һ10 mL��ˮϡ����100 mL���ӹ������ۣ������ʹ����ȫ��Ӧ��3TiO2+ +Al+6H+=3Ti3++Al3++3H2O�����˺�ȡ����Һ20.00 mL�������еμ�2��3��KSCN��Һ��ָʾ������ ����һ�ֲ������������ƣ��μ�0.1000mol��L-1 FeCl3��Һ������Һ���ֺ�ɫ�ﵽ�ζ��յ㣬��ȥ��30.00mL FeC13��Һ��������Һ��TiOSO4�����ʵ���Ũ���� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com