



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
A£®pH=1µÄČÜŅŗ£ŗK ”¢Cu ”¢Cu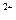 ”¢Br ”¢Br ”¢SO ”¢SO |
B£®ŗ¬ÓŠ0.1 mol”¤L I I µÄČÜŅŗ£ŗNH µÄČÜŅŗ£ŗNH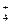 ”¢Fe ”¢Fe ”¢SO ”¢SO ”¢Cl ”¢Cl |
C£®ŗ¬ÓŠ0.1 mol”¤L HCO HCO µÄČÜŅŗ£ŗNa µÄČÜŅŗ£ŗNa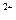 ”¢Al ”¢Al ”¢NO ”¢NO ”¢OH ”¢OH |
D£®ĒæĖįŠŌČÜŅŗ£ŗK ”¢Mg ”¢Mg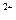 ”¢NO ”¢NO ”¢ClO ”¢ClO |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ĒæĖįŠŌČÜŅŗÖŠ£ŗAl3+”¢K+”¢Mg2+”¢Cl£”¢SO42£ |
| B£®PH=13µÄČÜŅŗÖŠ£ŗCl££¬HCO3££¬NO3££¬NH4+ |
| C£®c(Fe3+)="0.1" mol”¤L-1µÄČÜŅŗÖŠ£ŗNa+”¢SCN”Ŗ”¢Cl£”¢Br£ |
| D£®Ēæ¼īŠŌČÜŅŗÖŠ£ŗClO£”¢SO42£”¢SO32£”¢Na+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Ź¹pHŹŌÖ½³ŹŗģÉ«µÄČÜŅŗÖŠ£ŗFe2+”¢ClO-”¢SO42-”¢Na+ |
| B£®c(H+)=1.0”Į10-13 mol/L µÄČÜŅŗÖŠ£ŗNa+”¢SO42-”¢Al O2-”¢CO32- |
| C£®¼ÓČėĀĮ·ŪŗóÄܲśÉś“óĮæĒāĘųµÄČÜŅŗÖŠ£ŗNH4£«”¢Na£«”¢NO3-”¢SO42- |
| D£®ÓÉĖ®µēĄė³öc£ØH+£©=10-12 mol”¤L-1µÄČÜŅŗ£ŗK+”¢Al3+”¢Cl-”¢SO42- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Ė®½ā“ļµ½Ę½ŗā(²»±„ŗĶ)Ź±£¬ĪŽĀŪ¼ÓĀČ»ÆĢś±„ŗĶČÜŅŗ»¹ŹĒ¼ÓĖ®Ļ”ŹĶ£¬Ę½ŗā¾łĻņÕż·½ĻņŅĘ¶Æ |
| B£®ÅضČĪŖ5 mol/LŗĶ0.5 mol/LµÄĮ½ÖÖFeCl3ČÜŅŗ£¬ĘäĖūĢõ¼žĻąĶ¬Ź±£¬Fe3£«µÄĖ®½ā³Ģ¶ČĒ°Õß±ČŗóÕߊ” |
| C£®ÓŠ50”ęŗĶ20”ęµÄĶ¬ÅØ¶ČµÄFeCl3ČÜŅŗ£¬ĘäĖūĢõ¼žĻąĶ¬Ź±£¬Fe3£«µÄĖ®½ā³Ģ¶ČĒ°Õß±ČŗóÕߊ” |
| D£®ĪŖŅÖÖĘFe3£«Ė®½ā£¬½ĻŗƵŲ±£“ęFeCl3ČÜŅŗ£¬Ó¦¼ÓÉŁĮæŃĪĖį |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
A£®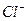 ”¢ ”¢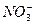 ”¢ ”¢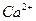 ”¢ ”¢ | B£®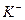 ”¢ ”¢ ”¢ ”¢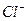 ”¢ ”¢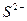 |
C£® ”¢ ”¢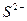  ”¢ ”¢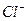 ”¢ ”¢ | D£® ”¢ ”¢ ”¢ ”¢ ”¢ ”¢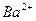 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Na+”¢NH4+”¢NO3-”¢Cl- | B£®K+”¢Na+”¢HCO3-”¢B- |
| C£®Na+”¢H+”¢NO3-”¢S- | D£®K+”¢Fe2+”¢NO3-”¢Cl- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ÄÜŹ¹ŹÆČļ³ŹĄ¶É«µÄČÜŅŗÖŠ£ŗ Na+”¢I£”¢Cl£”¢NO3- |
| B£®ÄÜŹ¹¼×»ł³Č³Ź»ĘÉ«µÄČÜŅŗÖŠ£ŗK£«”¢SO32-”¢SO42-”¢ClO£ |
| C£®ÄÜŹ¹pHŹŌÖ½±äŗģÉ«µÄČÜŅŗÖŠ£ŗMg2+”¢Fe3+”¢NO3-”¢[Ag(NH3)2]+ |
D£®ĪŽÉ«ČÜŅŗÖŠ£ŗK£«”¢Cl£”¢NO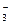 ”¢SO ”¢SO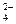 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Na£«”¢NH4£«”¢£ŗHCO3Ņ»”¢OH£ |
| B£®Fe3£«”¢Zn2£«”¢I£”¢SO42£ |
| C£®H£«”¢K£«”¢NO3£”¢SO42Ņ» |
| D£®Ca2£«”¢Na£«”¢NO3£”¢CO32£ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com