
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������Һ��c(CH3COO��)=c(Na+)ʱ����������������ǡ����ȫ��Ӧ |
| B������Һ��c(CH3COO��)=c(Na+)ʱ��һ�����������ƹ��� |
| C������Һ��c(CH3COO��)=c(Na+)��c(H+)=c(OH��)ʱ��һ���Ǵ������ |
| D������Һ��c(Na+)��c(CH3COO��)��c(OH��)��c(H+)ʱ��һ�����������ƹ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��KOH(aq)+1/2H2SO4(aq)==1/2K2SO4(aq)+H2O(l); ��H��-11.46 kJ/mol |
| B��2KOH(aq)+ H2SO4(aq)==K2SO4(aq)+2H2O(g);��H��-114.6 kJ/mol |
| C��2KOH(aq)+ H2SO4(aq)==K2SO4(aq)+2H2O(l);��H��+114.6 kJ/mol |
| D��KOH(aq)+1/2H2SO4(aq)==1/2K2SO4(aq)+H2O(l);��H��-57.3 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
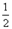 O2(g) ��H1 =" +242" kJ��mol-1
O2(g) ��H1 =" +242" kJ��mol-1 O2(g)="MgO(s) " ��H3 = -602kJ��mol-1
O2(g)="MgO(s) " ��H3 = -602kJ��mol-1
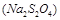 �����ã� ��
�����ã� ��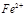 ��Ӧ����������ˮ�����ʣ�����
��Ӧ����������ˮ�����ʣ�����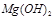 ��Ӧ����Ȼ
��Ӧ����Ȼ ������ˮ���������������EDTA�ļ��룬�����ܹ���
������ˮ���������������EDTA�ļ��룬�����ܹ��� ��ȥ����ô��ȸߵ�
��ȥ����ô��ȸߵ� ����ӳ����ܽ�ƽ��ĽǶȼ��Խ��� ��
����ӳ����ܽ�ƽ��ĽǶȼ��Խ��� ��| ������ȼ�������� | ��ȼ�������� | |||
| ��� | �ᴿ��ϵ�¶�/�� | ����EDTA����/g | ���뱣�շ�����/g | W(Fe)/(10-4g) |
| 1 | 40 | 0.05 | 0.05 | 7.63 |
| 2 | 40 | 0.05 | 0.10 | 6.83 |
| 3 | 60 | 0.05 | 0.10 | 6.83 |
| 4 | 60 | 0.10 | 0.10 | 6.51 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2NH3(g)�����仯����ͼ���ش��������⣺
2NH3(g)�����仯����ͼ���ش��������⣺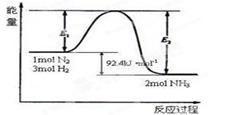
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��C2H5OH��l��+3O2��g��==2CO2��g��+3H2O��g������H=��1367��0 kJ/mol��ȼ���ȣ� |
| B��NaOH��aq��+HCl��aq��==NaCl��aq��+H2O��l������H=+57��3kJ/mol���к��ȣ� |
| C��S��s��+O2��g��===SO2��g������H=��269��8kJ/mol����Ӧ�ȣ� |
| D��2NO2==O2+2NO����H=+116��2kJ/mol����Ӧ�ȣ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2NH3(g) ��H��a kJ/mol���Ը��ݱ������м������ݹ���a����ֵ�� ��
2NH3(g) ��H��a kJ/mol���Ը��ݱ������м������ݹ���a����ֵ�� ��| ��ѧ�� | H��H | N��H | N��N |
| ���ܣ�kJ/mol�� | 436 | 391 | 945 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CH4 (g)+2NO2 (g)= N2 (g)+CO2 (g)+2H2O (l) ����H���D867 kJ��mol��1 |
| B����0.2 mol CH4��ԭNO2��N2�������������·ų�������Ϊ173.4 kJ |
| C��CH4����ԭNOxΪN2�Ĺ����У���x=1.6����ת�Ƶĵ���Ϊ3.2 mol |
| D�����ñ�״����2.24L CH4��ԭNO2��N2������������ת�Ƶĵ���Ϊ1.6 mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 0�֣�����β���к���CO��NO2
0�֣�����β���к���CO��NO2 ���ж����壬��������װβ������װ�ã���ʹ�ж��������Ӧת���������塣
���ж����壬��������װβ������װ�ã���ʹ�ж��������Ӧת���������塣 (g) ��H����393.5 kJ��mol-1
(g) ��H����393.5 kJ��mol-1 ��H�� �� �����ڸ÷�Ӧ���¶Ȳ�ͬ��T2��T1��������������ͬʱ������ͼ����ȷ���� ��
��H�� �� �����ڸ÷�Ӧ���¶Ȳ�ͬ��T2��T1��������������ͬʱ������ͼ����ȷ���� ��  ������ţ���
������ţ����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com