
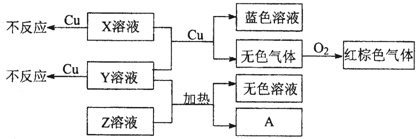
�ס��ҡ�����������Ϊԭ��������������Ķ�����Ԫ�ء��ס�������ͬһ���壬���������촦��ͬһ���ڣ���ԭ�ӵ������������Ǽס��ҡ���ԭ������������֮�͡��ס�����ɵij�������X��ʹʪ��ĺ�ɫʯ����ֽ��������ĵ�����X��Ӧ�������ҵĵ��ʣ�ͬʱ�õ�һ�ְ���Y��һ ��ǿ��Z�����ĵ��ʼ������Ԫ������������ˮ�������Һ��Ӧ������LҲ����Z��ˮ��Һ��Ӧ�����Σ����������ɻ�����M����ش���������
��1�������ӵĽṹʾ��ͼΪ_______��
��2��д���Һ�Y�ĵ���ʽ��_______��___________��
�õ���ʽ��ʾZ���γɹ���________________________________
��3����ĵ�����X��Ӧ���ɵ�Y��Z�����ʵ���֮��Ϊ2:4����Ӧ�б������������뱻��ԭ�����ʵ����ʵ���֮��Ϊ________��
��4��д������Z��ϡ��Һ�������L��ϡ��Һ�з�����Ӧ�����ӷ���ʽ��
��1��Cl����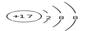 ��2��
��2��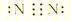

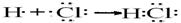
��3��2��3 ��4��H����AlO2����H2O=Al(OH)3��
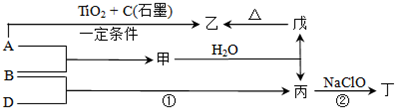
��������
����������ס�����ɵij�������X��ʹʪ��ĺ�ɫʯ����ֽ������˵��������ΪNH3���Դ�Ϊͻ�ƿڣ����ƶϳ����ұ�����ֱ�ΪH��N��Na��Al��Cl,XΪNH3��YΪNH4Cl��ZΪHCl��LΪNaAlNO3��MΪNaCl��
��1��Ϊ�����ӵĽṹʾ��ͼ��
��2��Ϊ�������Ȼ�淋ĵ���ʽ���Ȼ�����γɹ��̡�
��3���˷���ʽΪ3Cl2��4NH3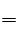 2NH4Cl=4HCl��N2�����У�3mol�����������˻�ԭ��Ӧ��2mol����������������Ӧ���ʱ����������ʺͱ���ԭ�����ʵ����ʵ���֮��Ϊ2:3.
2NH4Cl=4HCl��N2�����У�3mol�����������˻�ԭ��Ӧ��2mol����������������Ӧ���ʱ����������ʺͱ���ԭ�����ʵ����ʵ���֮��Ϊ2:3.
���㣺����ʽ���йض�����Ԫ�ص�����
����������ͨ����Ԫ�����ڱ������Ԫ�ص��ƶϣ����������ʣ��漰����ʽ���ṹʽ����д�Լ���������ԭ��Ӧ����������ԭ�����жϣ��Ѷ��еȡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ���¸�ѹ |
| ||
| ���¸�ѹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ���� |
| ���¸�ѹ |
| ���� |
| ���¸�ѹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

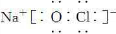
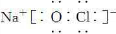
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


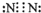
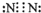
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������Ӱ뾶�����������ң��� | B����̬�⻯����ȶ��ԣ��ף��� | C��������γɵĻ��������һ�� | D�����������������������Ӧ��ˮ����֮���������ܷ�Ӧ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com