
��32.64gͭ��140mLһ��Ũ�ȵ����ᷴӦ��ͭ��ȫ�ܽ������NO��NO2��������ڱ�״���µ����Ϊ11.2L����ش�
��1��NO�����Ϊ L��NO2�����Ϊ L��
��2��������������ȫ���ͷź�����Һ����VmL a mol/L��NaOH��Һ��ǡ��ʹ��Һ�е�Cu2��ȫ��ת���ɳ�������ԭ������Һ��Ũ��Ϊ mol/L��
��3����ʹͭ�����ᷴӦ���ɵ�������NaOH��Һ��ȫ��ת��ΪNaNO3��������Ҫ30%��˫��ˮ
g��
��1��5.8 5.4 ��2�� (av.10-3+0.5)/0.14 ��3��57.67
��������
���������ͭ��һ��Ũ�ȵ����ᷴӦ���漰�Ļ�ѧ����ʽΪ��
Cu+4HNO3(Ũ)=Cu(NO3)2+2H2O+NO2 ��3Cu+8HNO3(ϡ)=3Cu(NO3)2+4H2O+2NO
��3Cu+8HNO3(ϡ)=3Cu(NO3)2+4H2O+2NO
��1����NO�����Ϊ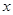 L����NO2�����Ϊ
L����NO2�����Ϊ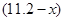 L������������ʽ��֪����ϡ���ᷴӦ��ͭ������Ϊ
L������������ʽ��֪����ϡ���ᷴӦ��ͭ������Ϊ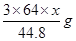 ������Ũ���ᷴӦ��ͭ������Ϊ
������Ũ���ᷴӦ��ͭ������Ϊ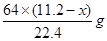 ����˿ɵ����µ�ʽ��
����˿ɵ����µ�ʽ�� �����
�����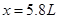 �����NO�����Ϊ5.8L��NO2�����Ϊ5.4L��
�����NO�����Ϊ5.8L��NO2�����Ϊ5.4L��
��2����Һ��ͭ���ӵ����ʵ��� �����ԭ��������ʵ���Ũ��
�����ԭ��������ʵ���Ũ��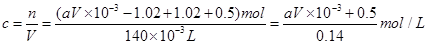
��3�������ķ�ӦΪ2NO2+H2O2=2HNO3��2NO+3H2O2=2HNO3+2H2O������NO2��Ӧ��H2O2������Ϊ ����NO��Ӧ��H2O2������Ϊ
����NO��Ӧ��H2O2������Ϊ ����ɵ����¹�ϵʽ��
����ɵ����¹�ϵʽ�� �����
�����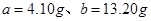 �������Ҫ30%��˫��ˮ������Ϊ
�������Ҫ30%��˫��ˮ������Ϊ ��
��
���㣺ͭ������ķ�Ӧ
���������⿼����ͭ�����ᷴӦ�����漰���ļ��㣬���ڻ����⡣����Ĺؼ�������ȷд����ѧ����ʽ���ڼ���Ĺ�����Ӧע���ϵʽ�����غ㷨��Ӧ�á�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| aV��10-3+0.5 |
| 0.14 |
| aV��10-3+0.5 |
| 0.14 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
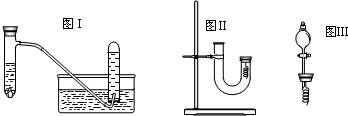
| ʵ�鲽�� | ���� |
| 1��U����˼���ϡ����ֱ������U���Ҷ� | ��/ |
| 2�ø���ͭ˿�Ľ�����סU���Ҷˣ��۲����� | ������ ����ɫ����������ұ���Һ�����ɫ ����ɫ����������ұ���Һ�����ɫ |
| 3����Ӧֹͣ��������۲�ʵ������ | ������ ��ɫ����������Ӵ���������ɺ���ɫ ��ɫ����������Ӵ���������ɺ���ɫ |
| 10-3a�qV +0.5 |
| 0.14 |
| 10-3a�qV +0.5 |
| 0.14 |
| 1 |
| 2 |
|
|
| 1 |
| 2 |
|
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ������һ�и�����һ��������⻯ѧ�Ծ����������� ���ͣ�������
�����dz���������ǿ��֮һ���ڻ�ѧ�о��ͻ������������Ź㷺Ӧ�ã��������Ʊ������Ρ�Ⱦ�ϡ����ϡ�ҽҩ�м��塢����ըҩ�ȡ������ζ���������Լ���ͼ������ҵ��
��1��ij����M������������ʱ����ʽ�ֽ⣺2MNO3 2M+2NO2��+O2��������3.40gMNO3������NO2��O2����ɱ�״��ʱ�������Ϊ672mL���ɴ˿��Լ����M�����ԭ������Ϊ_____________��
2M+2NO2��+O2��������3.40gMNO3������NO2��O2����ɱ�״��ʱ�������Ϊ672mL���ɴ˿��Լ����M�����ԭ������Ϊ_____________��
��2����32.64gͭ��140mL һ��Ũ�ȵ����ᷴӦ��ͭ��ȫ�ܽ������NO��NO2�����������ɱ�״���µ����Ϊ11.2L������NO�����Ϊ_____________��
��3������Cu��Cu2O��CuO��ɵĻ���ij�о���ѧϰС��Ϊ��̽����������������100mL0.6mol/LHNO3��Һǡ��ʹ�������ȫ�ܽ⣬ͬʱ�ռ���224mLNO���壨��״�����������������ͭ�����ʵ���Ϊ ����ԭ���������0.0lmolCu��������Cu2O��CuO��������Ϊ_____________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com