
 AŹĒŅ»ÖÖ°×É«¾§Ģ壬ĖüÓėÅØĒāŃõ»ÆÄĘČÜŅŗ¹²ČČ£¬·Å³öĪŽÉ«ĘųĢåB£®ÓĆŌ²µ×ÉÕĘæŹÕ¼ÆøÉŌļµÄB£®°“ČēĶ¼×°ÖĆŅĒĘ÷£¬¼·Ń¹µĪ¹ÜµÄ½ŗĶ·Ź±£¬æÉŅŌµĆµ½Ą¶É«ÅēČŖ£»AÓėÅØĮņĖį·“Ó¦£¬·Å³öĪŽÉ«ĘųĢåC£¬CŌŚæÕĘųÖŠÄÜŠĪ³É°×Īķ£®ÓĆŌ²µ×ÉÕĘæŹÕ¼ÆøÉŌļµÄC£¬ČŌ°“ČēĶ¼×°ÖĆŅĒĘ÷£¬¼·Ń¹µĪ¹ÜµÄ½ŗĶ·Ź±£¬æÉŅŌµĆµ½ŗģÉ«ÅēČŖ£®
AŹĒŅ»ÖÖ°×É«¾§Ģ壬ĖüÓėÅØĒāŃõ»ÆÄĘČÜŅŗ¹²ČČ£¬·Å³öĪŽÉ«ĘųĢåB£®ÓĆŌ²µ×ÉÕĘæŹÕ¼ÆøÉŌļµÄB£®°“ČēĶ¼×°ÖĆŅĒĘ÷£¬¼·Ń¹µĪ¹ÜµÄ½ŗĶ·Ź±£¬æÉŅŌµĆµ½Ą¶É«ÅēČŖ£»AÓėÅØĮņĖį·“Ó¦£¬·Å³öĪŽÉ«ĘųĢåC£¬CŌŚæÕĘųÖŠÄÜŠĪ³É°×Īķ£®ÓĆŌ²µ×ÉÕĘæŹÕ¼ÆøÉŌļµÄC£¬ČŌ°“ČēĶ¼×°ÖĆŅĒĘ÷£¬¼·Ń¹µĪ¹ÜµÄ½ŗĶ·Ź±£¬æÉŅŌµĆµ½ŗģÉ«ÅēČŖ£®
| ||
| ||
| ||
| ||



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 µŖ¼°Ęä»ÆŗĻĪļŌŚ
µŖ¼°Ęä»ÆŗĻĪļŌŚ ¹¤Å©ŅµÉś²śµČ·½ĆęÓŠ¹ć·ŗÓ¦ÓĆ£¬ŃŠ¾æĘäŠŌÖŹÓŠÖŲŅŖŅāŅ壮
¹¤Å©ŅµÉś²śµČ·½ĆęÓŠ¹ć·ŗÓ¦ÓĆ£¬ŃŠ¾æĘäŠŌÖŹÓŠÖŲŅŖŅāŅ壮²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

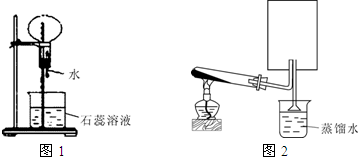
£Ø1£©AµÄĖ®ČÜŅŗĻŌČõĖįŠŌ£¬ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾ĘäŌŅņ_____________________________”£
£Ø2£©ĘųĢåBµÄøÉŌļ¼ĮŹĒ__________________£¬°“ÉĻĶ¼£Øb£©×°ŗĆŅĒĘ÷£¬ŹÕ¼ÆĘųĢåBµÄ·½·ØŹĒ
___________________________________________ӣ
¼ģŃéĘųĢåBŅŃ¼ÆĀśµÄ·½·ØŹĒ________________________________”£
£Ø3£©øÉŌļµÄĄ¶É«ŹÆČļŹŌÖ½Óöµ½øÉŌļµÄĘųĢåC²»±äÉ«µÄŌŅņŹĒ______________________”£
£Ø4£©¼ÓČČŹ±£¬¼×Ėį“ß»ÆĶŃĖ®æÉÖʵĆŅ»Ńõ»ÆĢ¼”£·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ___________________”£
øł¾ŻÕāøö·“Ó¦ÖĘČ”Ņ»Ńõ»ÆĢ¼µÄ·¢Éś×°ÖĆÓėÖĘČ”ĘųĢå_________£ØĢī”°B”±»ņ”°C”±£©µÄ×°ÖĆĻąĶ¬”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğø£½ØŹ”ÄĻ°²Ņ»ÖŠøßŅ»ĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
µŖ¼°Ęä»ÆŗĻĪļŌŚ¹¤Å©ŅµÉś²śµČ·½ĆęÓŠ¹ć·ŗÓ¦ÓĆ£¬ŃŠ¾æĘäŠŌÖŹÓŠÖŲŅŖŅāŅ唣
AŹĒŅ»ÖÖ°×É«¾§Ģ壬ĖüÓėÅØNaOHČÜŅŗ¹²ČČ£¬·Å³öĪŽÉ«ĘųĢåB”£ÓĆŌ²µ×ÉÕĘæŹÕ¼ÆøÉŌļµÄB”£°“ČēĶ¼×°ÖĆ£¬Ņż·¢×ĻÉ«ŹÆČļČÜŅŗÉĻÅē£¬æÉŅŌµĆµ½Ą¶É«ÅēČŖ£»AÓėÅØH2SO4·“Ó¦£¬·Å³öĪŽÉ«ĘųĢåC”£ÓĆŌ²µ×ÉÕĘæŹÕ¼ÆøÉŌļµÄC£¬ČŌ°“ĻĀĶ¼×°ÖĆ£¬Ņż·¢×ĻÉ«ŹÆČļČÜŅŗÉĻÅē£¬æÉŅŌµĆµ½ŗģÉ«ÅēČŖ”£
£Ø1£©Š“³öAÓėÅØNaOHČÜŅŗ¹²ČČÉś³ÉBµÄĄė×Ó·½³ĢŹ½
£»
£Ø2£©æÉÓĆÓŚ³żČ„BÖŠĖ®·ÖµÄøÉŌļ¼ĮŹĒ______________£»
ŹÕ¼ÆĘųĢåCµÄ·½·ØŹĒ______________________________£»
£Ø3£©Ņż·¢×ĻÉ«ŹÆČļČÜŅŗÉĻÅēµÄ²Ł×÷ŹĒ £»
£Ø4£©ŌŚĶ¬ĪĀĶ¬Ń¹ĻĀ£¬ĻąĶ¬Ģå»żµÄĮ½øöÉÕĘæ·Ö±š³äĀśĘųĢåBŗĶĘųĢåC£¬×öÅēČŖŹµŃéŗó£¬Ė®¶¼³äĀśÉÕĘ棬Į½øöÉÕĘæÖŠĖłµĆČÜŅŗµÄĪļÖŹµÄĮæÅضČÖ®±ČŹĒ_____________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com