
���淴Ӧ��2NO22NO+O2�ں��º����ܱ������з�Ӧ���ﵽƽ��״̬�ı�־��
�ٵ�λʱ��������n molO2��ͬʱ����2n molNO2
�ڵ�λʱ��������n molO2 ��ͬʱ����2n mol NO
����NO2��NO��O2 �����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ���ʵı�Ϊ2 2 1��״̬
�ܻ���������ɫ���ٸı��״̬
�ݻ��������ܶȲ��ٸı��״̬
��������ƽ����Է����������ٸı��״̬
A���٢ܢ� B���ڢۢ� C���٢ۢ� D���٢ڢۢܢݢ�
 �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪XԪ��ԭ�ӵ�L���Ӳ��YԪ��ԭ�ӵ�L���Ӳ���3�����ӣ�YԪ��ԭ�Ӻ����ܵ�������XԪ��ԭ���ܵ�������5������ش��������⣺
(1)д����Ԫ�ص����ƣ�X________��Y________��
(2)X��Y���γ�________��
A�����ӻ�����Y3X2 B�����ӻ�����Y2X3
C�����ۻ�����Y3X2 D�����ۻ�����XY2
(3)��֪YԪ�صĵ��ʣ����ڿ�����ȼ�գ�д�����������û���Ӧ�Ļ�ѧ����ʽ__________________���������õ�����������ѧ��������________��
(4)XԪ�ص��⻯��������������������ˮ���ﷴӦ�����������________�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й���ҩƷ�ı��淽����ȷ����(����)
A�������Ʊ�����������
B����̬��ʢ��ϸ�ڲ���ƿ��
C��Һ�屣���ڼ�������ˮ�ĸ��в���������ɫϸ��ƿ��
D��ŨHNO3ʢ�ڸ�����������ɫƿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����(����)
����Ҫ���ȷ��ܷ����ķ�Ӧһ�������������ķ�Ӧ
���ͷ������ķ�Ӧ�ڳ�����һ����������
�۷�Ӧ���ͷ��������������������뿴��Ӧ��������������е�����������Դ�С
���ͷ������ķ�Ӧ���ȵ�һ���¶�������ֹͣ���ȷ�ӦҲ�ܼ�������
A��ֻ�Тۢ� B��ֻ�Т٢�
C���٢ڢۢ� D��ֻ�Тڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ�����Թܷ���ʢ��25 ��ʱ����ʯ��ˮ���ձ��У��Թ��п�ʼ���뼸С��þƬ���ٵ���5 mL���ᣬ�Իش��������⡣
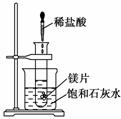
(1)ʵ���й۲쵽��������_________________________________________________
________________________________________________________________________��
(2)�������������ԭ����__________________________________________________
________________________________________________________________________
________________________________________________________________________��
(3)д���йط�Ӧ�����ӷ���ʽ��___________________________________________��
(4)��ʵ����֪��MgCl2��Һ��H2��������________(����ڡ�����С�ڡ����ڡ�)þƬ���������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ����
A�������ΪVa��0.02mol��L-1CH3COOH��Һ�м������ΪVb��0.02mol��L-1NaOH��Һ��Va��Vbʱ��c(CH3COOH)+c(CH3COO-)��c (Na+)
B����0.2 mol��L-1��������0.1 mol��L-1��KAlO2��Һ�������ϣ�����Һ������Ũ����С�����˳��Ϊ��c(OH-)��c(Al3+)��c(H+)��c(K+)��c(Cl-)
C��pH��5��HCOOH��Һ��pH��5��NH4NO3��Һ�У�c(H+)�����
D��25��ʱ��pH��4��Ũ�Ⱦ�Ϊ0.1mol��L-1��CH3COOH��CH3COONa�����Һ��c(CH3COO-)��c(OH-)��c(CH3COOH)��c(H+)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪NaHSO3��Һ�����ԣ���Һ�д�������ƽ�⣺
HSO3�D+ H2OH2SO3 + OH�D �� HSO3�D
H+ + SO32�D ��
��0.1 mol �� L -1��NaHSO3��Һ�зֱ�����������ʣ������й�˵����ȷ����
A��������������Na��ƽ������ƣ�ƽ������ƣ���Һ��c(HSO3�D)����
B����������Na2SO3���壬��c(H+) + c(Na+) = c(HSO3�D) + c(OH�D) +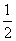 c(SO32�D)
c(SO32�D)
C����������NaOH��Һ��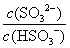 ��
��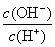 ��ֵ������
��ֵ������
D�����백ˮ�����ԣ���2c(Na+) = c(SO32�D)��c(H+) = c(OH�D)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʲ���ͨ�����Ϸ�Ӧ�õ�����
A��FeCl2 B��Fe(OH)3 C��H2SiO3 D��NaHSO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һ�ֹ��廯����X��X���������磬������״̬������ˮʱ�ܹ����룬���й��ڸû�����X��˵���У���ȷ����(����)
A��Xһ��Ϊ����� B��X����Ϊ�ǵ����
C��Xֻ�������� D��X�������κλ�����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com