
ijͬѧ������֪���ʵ���Ũ��Ϊ0.100 0 mol��L-1������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ���̪��ָʾ��������д���пհף�
(1)�ñ�������ζ����������������Һʱ�����ְ�����ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע����ƿ����Һ��ɫ�ı仯������жϵζ��յ㣺 ��
(2)���в����п���ʹ��������������Һ��Ũ����ֵƫ�͵��� (�����)��
A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
B���ζ�ǰʢ������������Һ����ƿ������ˮϴ�� ��û�и���
��û�и���
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
E���ζ������У���ƿ�����ڼ��ң�ʹ������Һ����
(3)����һ�εζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ������ͼ��ʾ������ʼ����ΪV1= mL���յ����V2= mL��
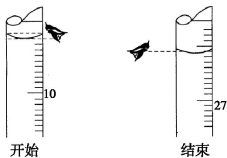
(4)�ٽ���±����ݣ����㱻������������Һ�����ʵ���Ũ���� mol��L-1��
�ζ����� | ������Һ���/mL | ������� | |
�ζ�ǰ�Ŀ̶�/mL | �ζ���Ŀ̶�/mL | ||
��һ�� | 10.00 | V1 | V2 |
�ڶ��� | 10.00 | 4.10 | 21.10 |
������ | 10.00 | 0.40 | 17.60 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꺣��ʡ�߶��������Ļ�ѧ���������棩 ���ͣ������
��1��������ʳ��Ӧ�����е�Ӫ���ذ������ࡢ________�������ʡ�________��ˮ�Ϳ����ʵȡ�
��2������ˮ�����ղ�����________������ˮ������ˮ�������__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����������·һ�и߶��϶ο��Ļ�ѧ���������棩 ���ͣ�ѡ����
���й�����ϩ��˵����ȷ����
A������Ȼ������Ҫ�ɷ� B����ʹ������Ȼ�̼��Һ��ɫ
C�����ܷ���ȼ�շ�Ӧ D�����ܷ����Ӿ۷�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����������·һ�и߶��϶ο��Ļ�ѧ���������棩 ���ͣ�ѡ����
Ư�۵���Ч�ɷ���
A��Ca(ClO)2 B��CaCl2 C��Ca(OH)2 D��CaCO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����������·һ�и߶��϶ο��Ļ�ѧ���������棩 ���ͣ�ѡ����
���г���������漰��ѧ�仯����
A����ˮ�ɱ� B����ɳ���� C�����Ͻ��� D������ĥ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����������·һ�и߶��϶ο�����ѧ���������棩 ���ͣ�ѡ����
��������ĵ���ƽ�ⳣ�����±���
���� | HCOOH | HCN | H2CO3 |
����ƽ�ⳣ��(25 ��) | Ki=1.77��10-4 | Ki=4.9��10-10 | Ki1=4.3��10-7 Ki2=5.6��10-11 |
����ѡ��������( )
A��2CN-+H2O+CO2=2HCN+ CO32-
B��2HCOOH+ CO32-=2HCOO-+H2O+CO2��
C���к͵���� ����c(H+)��HCOOH��HCN����NaOH����ǰ��С�ں���
����c(H+)��HCOOH��HCN����NaOH����ǰ��С�ں���
D����ͬ���ʵ���Ũ�ȵ�HCOOH��HCN��Һ�У�c( HCOO-)<c(CN-)
HCOO-)<c(CN-)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����������·һ�и߶��϶ο�����ѧ���������棩 ���ͣ�ѡ����
25�桢101 kPa�£�̼������������������ǵ�ȼ����������393.5 kJ/mol��285.8 kJ/mol��890.3kJ/mol��2800 kJ/mol���������Ȼ�ѧ����ʽ��ȷ���� ( )
A��C(s)+1/2 O2(g)=CO(g)����H= - 393.5 kJ/mol
O2(g)=CO(g)����H= - 393.5 kJ/mol
B��2H2(g)+O2(g)=2H2O(l)����H=+571.6 kJ/mol
C��CH4(g)+2O2(g)=CO2(g)+2H2O(g)����H=-890.3 kJ/mol
D��1/2C6H12O6(s)+3O2(g)=3CO2(g)+3H2O(l)����H=-1400 kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ��һ�����л�ѧ���������棩 ���ͣ�ѡ����
���г��������뽺���ص���
A���峿�������п���һ���ƵĹ���
B�����м��������ʹ�����ʵȾ۳����ƳɿɿڵĶ���
C��FeCl3��Һ����NaOH��Һ�������ɫ����
D�����õ�Ӿ�����ᡢ�齺���������ȵس����ڶƼ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017������ʦ���и������¿�����ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵����ȷ����
A�����������������ԭ�ӱ���ԭ��ȡ���������ֲ�ͬ�ṹ����
B����ȩ��ʹ�����ʱ��ԣ�������ʳƷ������
C���״�������ˮ����֮����������ʹ�״���ˮ����������ǿ
D���ױ��뱽��Ϊͬϵ�����ʹKMnO4������Һ��ɫ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com