
��15�֣�ijУһ�о���ѧϰС��Ե����������ȷֽ�������ۡ�
�����Dz������ϵ�֪���������������ں����м���ʱ����79��134��,����ʧ��14��4����134��250�棬��ʧ��14��4����250��300�棬��ʧ��7��2����֮�������620�棬����ά�ֲ��䡣С�龭������ó���������300��620��Ĺ��������Ϊ��ˮ����ͭ��134��ʱ�Ĺ��������Ļ�ѧʽΪ ��
��С�����ˮ����ͭ�������¼��ȵĿ��ܱ仯���в��롣��������˲�������¼��ֲ��룺
�٣�CuO��SO3����
�ڣ�CuO��SO2��O2��
�ۣ�CuO��SO3��SO2��
�ܣ�CuO��SO3��SO2��O2
С�龭���������ۣ���Ϊ����۲���ʵ��Ϳ��ų������ǵ�������
�������ϣ���SO3Ϊ��ɫ���壬�۵�16��6�棬�е�44��8�档
��SO2���۵�:��72��4�棬�е�:��10�棻SO2ͨ��BaCl2��Һ�У�����������
��ʵ��̽����
С�鰴��ͼ��ʾ��װ��ʵ��װ�á�
��1����װ��װ�ú�δװҩƷǰ������еIJ����� ��
Dװ�õ������� ��
��2����ͼʾװ��ҩƷ���þƾ���ƶ���Ӳ���Թܼ��ȡ�һ�����B����Һ������ɫ���ǣ�C����Һ����ɫ��
����ʵ����������
��1��С��ͬѧ�����Ϊ��ˮ����ͭ�ȷֽ����Ӧ��Ϊ����ܡ�����һ��ͬѧ������ɣ�����ΪB����Һ������ɫ���Dz���һ����ȷ�������к���SO3�����������漰�Ļ�ѧ����ʽ�� �����ǣ�С��ͬѧ�����۾�����������һ��װ��E������Ϊ��װ��Ӧ���� ����װ����ĸ��֮�䡣����װ�ú�С������ʵ�飬֤���˲�����ȷʵ����SO3������Ϊ���Ǹ���ʲô����õ���һ���ۣ� ��
��2��С���������ˮ����ͭ�ȷֽ�Ļ�ѧ����ʽʱ���������ѡ����Ƿ��ָû�ѧ����ʽΪ��������ʽ��������������ƽ������������ܵط�����������ΪֻҪ��ȷ��ijЩ���ʵļ�����֮�ȣ�����ȷ���û�ѧ����ʽ������֪SO2��SO3�ļ�����֮�ȣ�����ȷ���û�ѧ����ʽ������SO2��SO3�ļ�����֮��Ϊx����д����ƽ��Ļ�ѧ����ʽ ��
��CuSO4��3H2O
������ֻ�н���Ԫ�ض�������Ԫ�أ���Ԫ���غ�Ƕȷ���Ҳ�Ʒ֣�
��1������װ�������ԣ�1�֣� ����β������ֹ��Ⱦ�����������𰸾��Ʒ֣�
������1��2SO2+O2+2H2O=2H2SO4 H2SO4+BaCl2=BaSO4��+2HCl����һ����1�֣�д�ܷ�Ӧ����ʽ��2�֣�
A B���д���0�� �� E���Թ��������ɫ���壨дҺ��Ҳ���֣�
��2�� 2(x+1) CuSO4 ���� 2(x+1)CuO + 2SO3 ��+ 2xSO2 ��+ xO2��
�����������������������79��134��,����ʧ��14��4��������1mol���壬��ʧȥˮΪ250g��14��4%=36g����2molH2O��134��ʱ�Ĺ����������CuSO4��3H2O��
����CuSO4��5H2O��ΪCuO��SO3��SO2������SO2ʱS�Ļ��ϼ۽��ͣ�û��Ԫ�ػ��ϼ����ߣ��ʼ��費������
��1����װ��װ�ú�δװҩƷǰ������еIJ����Ǽ���װ�������ԡ�Dװ����ʢ��NaOH��Һ��������������β������ֹ��Ⱦ������
���㣺��ѧʵ����������ۡ�


 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����þ[Mg(ClO3)2]����������������ݼ��ȣ�ʵ�����Ʊ�����Mg(ClO3)2��6H2O���������£�
��֪����±����Ҫ�ɷ�ΪMgCl2��6H2O������MgSO4��FeCl2�����ʡ�
�����ֻ�������ܽ��(S)���¶�(T)�仯������ͼ��ʾ��
��1����������Ҫ����Ҫ���������� ��
��2������BaCl2��Ŀ���� ����MgO�����������������Ҫ�ɷ�Ϊ ��
��3������NaClO3������Һ������Ӧ�Ļ�ѧ����ʽΪ ���ٽ�һ����ȡMg(ClO3)2��6H2O��ʵ�鲽������Ϊ���������ᾧ���� ���� ���ܹ��ˡ�ϴ�ӡ�
��4����Ʒ��Mg(ClO3)2��6H2O�����IJⶨ��
����1��ȷ����3.50 g��Ʒ���100 mL��Һ��
����2��ȡ10.00 mL����ƿ�У�����10.00 mLϡ�����20 .00mL 1.000 mol��L��1��FeSO4��Һ���ȡ�
����3����ȴ�����£���0.100 mol��L��1 K2Cr2O7 ��Һ�ζ�ʣ���Fe2�����յ㣬�˹����з�Ӧ�����ӷ���ʽΪ��Cr2O72����6Fe2����14H����2Cr3����6Fe3����7H2O��
����4��������2��3�ظ����Σ�ƽ������K2Cr2O7 ��Һ15.00 mL��
��д������2�з�����Ӧ�����ӷ���ʽ�� ��
�ڲ�Ʒ��Mg(ClO3)2��6H2O����������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��ͼΪij��ʵ��װ��ʾ��ͼ�����м���װ�úͲ���ҩƷ�Ⱦ��Ѿ�ʡ�ԣ�װ�âٺ�װ�â�Ϊ���巢��װ�ã���
��1����ͬѧ�ô�װ�ã���A��B������һʢ��Ũ�����ϴ��ƿ���Ʊ�NO2����
֤�����ʡ�װ�â�����ʵ�����Ʊ�NH3����װ�â��Ʊ�����O2��װ�â��з���
��Ӧ�Ļ�ѧ����ʽ�� ��B����ˮ��ȴ��U�����к���ɫ��
��������Խ�ӽ�U�ܵײ���ɫԽdz�������ԭ�� ��
��2����ͬѧ�ô���װ�úϳ�SO3��B����ˮ��ȴ��U�����й�����֡�C��
��ʢ������K2Cr2O7��Һ������C�з�����������ԭ��Ӧ�����ӷ���ʽ�� ����������Cr��Cr3+��ʽ���ڣ��������Ƶô��������SO3����C��ʢ��NaOH��Һ�����װ���д������Բ��㣬Ӧ��θĽ� �������������ʵ��װ����ѡ����ĸ��Ų�����滻�����ü�Ҫ����˵��������滻��λ�ã���
��3����ͬѧ����֤NO�ܱ�������ԭ��������ת���ʣ�װ�â١�װ�âڷֱ���
NO��NH3����������װ��A��B��C�ֱ����Тݡ��ޡ��ߡ�
��ش�װ�âߵ����ÿ����� ��������װ�âݵ�NO��22.4L��������Ϊ��״������ͬ������������������ռ�����״����11.2LN2����NO��ת������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ������Ҫ�Ʊ��������Ȼ��ؾ��塣���к�����KBr��K2SO4���Ȼ�����Ʒ��������ͼ��ʾ��ʵ�鷽�������ᴿ��
��1������I������ ������������IJ��������оƾ��ƺ� ��
��2���������ᱵ�������Ȼ������Ƿ���У���˵�����ɡ�
��
��3����ʵ�����ù���ס��ҵ������ֱ�ΪW1g��W2g������Ʒ��KBr��������������ʽΪ ��
��4��ijͬѧ�Ը�ʵ�鷽��������ɣ�����Ϊ�������Ȼ�����Һ�������ƣ�Ӧ��������Ȼ�����Һ�����㰴����˼·��д��ʵ������ͼ�������Լ������ò���Ļ�ѧʽ����Ӧ���������ƣ�A ��B ����ҺC �������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��14�֣�������ع㷺�������������������������������������ⶾ�����������Ʊ��������£�
��֪��a��3MnO2+KClO3+6KOH��3K2MnO4+KCl+3H2O B��3K2MnO4��2CO2��2KMnO4��MnO2����2K2CO3
��ش��������⣺
��1��������з�Ӧ��ѡ�õ����������� ��
A��ʯӢ���� B�������� C�������� D��ʯī����
��2�������ͨCO2��Ŀ����Ϊ�˵�����Һ��pH���ܷ�����Ȼ��� ����ܡ�����ԭ���� ��
��3��������г��˵õ��Ĺ����� ��
��4������װ����Ҫ�� ����ȫƿ�������úͲ���©���IJ�����ɣ���ʵ���г���ʱ�ܷ�����ֽ��______����ܡ����ܡ����� ������_____��
��5������Һ�ݵõ�������صĺ�������������____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��15�֣�����ʯ�к��е���Ҫ��������������������ij��ѧ��ȤС���ô���ʯΪԭ����ȡ��ȫ��ɱ�����������Ƶ���Ҫ���̣�
��ش��������⣺
��1���Լ�A�������� ��
��2������I��Ŀ���� ��
��3����ʵ����Ҫ���ʹ�ò���������ʵ������в������������� ��
��4��д����Ӧ��������CaO2��8H2O�Ļ�ѧ����ʽ�� ��
��5���Ƶõ�CaO2��һ�㺬��CaO�û�ѧ��ȤС��ͨ��ʵ��ⶨ�Ƶõ���Ʒ��CaO2�ĺ���������0.6g��Ʒ����ƿ�У�Ȼ����������Ũ��Ϊ2.00mol?L-1������20.00mL������Ũ��Ϊ2.00mol?L-1������������Һ�ζ���ƿ�е���Һ����������������Һ11.00mL������Ʒ��CaO2����������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
һ��Ũ��NaHCO3��Һ��CuSO4��Һ��Ӧ������������ɫ����״�������ͳ����ɷ�������������ּ��裺
����һ��������CuCO3���������������Cu(OH)2��
��������������CuCO3��Cu(OH)2�Ļ���
(1)д���������������Cu(OH)2���ɵ����� (�����ӷ���ʽ��ʾ)��
(2)Ϊ��̽�������ijɷ֣�ȡ����һ���ֳ������μ�ϡ���ᣬ������ų���ƾ�������жϳ����к��� ��
(3)Ϊ�˽�һ��̽�������ijɷ֣�����ȷ�������к��ּ�����������ʵ�飬װ��ͼ���£�
���о����������ǰ���뽫��������Һ�з��벢�����������������Ϊ ��ϴ�ӡ����
��װ��E��ҩƷ�������� ����Ϊ ��
��ʵ������������²������裺a.��K1��K3���ر�K2��K4��ͨ������������˲���������� ��
b���ر�K1��K3����K2��K4����ַ�Ӧ��c.��ͨ���������ʱ���������ڴ��� ���رյ��� ��
����������Ʒ������Ϊm g��װ��D������������n g����������ƷΪ�����m�� n֮��Ĺ�ϵΪ ��
����������������Cu(OH)2����������Ϊ ���������в���c�����ʹ��ý�� (�ƫ�ߡ�����Ӱ�족��ƫ�͡�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�����к��зḻ�ĵ�Ԫ�أ���I����ʽ���ڣ���ʵ��������ȡ����������£�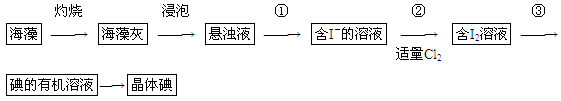 ��1��ʵ������۵�������________��������Ҫ��������Ϊ________��
��1��ʵ������۵�������________��������Ҫ��������Ϊ________��
��2����ȡ��Ĺ����У��ɹ�ѡ����л��Լ���________������ţ���
A���ƾ����е�78�棩 B�����Ȼ�̼���е�77�棩
C�����ͣ��е�290�棩 D�������е�80�棩
��3���ڲ������У���Һ����������ICl��ICl��������������������Ϊ���������ʣ�ʹ��ȫ�����������Ӧ��������������ţ�________��Һ����Ӧ�����ӷ���ʽ______________________��
A��KIO3 B��HClO C��KI D��Br2
��4�����õ���л���Һ�õ�����I2�ķ�����_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��14�֣��屽��һ�ֻ���ԭ�ϣ�ʵ���Һϳ��屽��װ��ʾ��ͼ���й��������£�
�����кϳɲ���ش����⣺
��1����a�м���15mL��ˮ����������м����b��С�ļ���4.0mLҺ̬�壬��a�е��뼸���壬�а�ɫ��������������Ϊ������ ���塣�����μ���Һ����꣬װ��d�������� ��
��2��Һ�����������в�������ᴿ��
����a�м���10mLˮ��Ȼ����˳�ȥδ��Ӧ����м��
����Һ������10mLˮ��8mL10%��NaOH��Һ��10mLˮϴ�ӡ�NaOH��Һϴ�ӵ������� ����ֳ��Ĵ��屽�м�����������ˮ�Ȼ��ƣ����á����ˣ������Ȼ��Ƶ��� ��
��3�������Ϸ���������屽�л����е���Ҫ����Ϊ ��Ҫ��һ���ᴿ�����в����б������ (������ȷѡ��ǰ����ĸ)��
A.�ؽᾧ B.���� C.���� D.��ȡ
��4���ڸ�ʵ���У�a���ݻ����ʺϵ��� (������ȷѡ��ǰ����ĸ)��
A.25mL B. 50mL C.250mL D.500mL
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com