
”¾ĪļÖŹ½į¹¹ÓėŠŌÖŹ”æ
ŅŌµŖ»ÆļŲ(GaN)ĪŖ“ś±ķµÄµŚČż“ś°ėµ¼Ģå²ÄĮĻÄæĒ°ŅŃ³ÉĪŖČ«Ēņ°ėµ¼ĢåŃŠ¾æµÄĒ°ŃŲŗĶČČµć”£»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ļŲĪŖŌŖĖŲÖÜĘŚ±ķµŚ31ŗÅŌŖĖŲ£¬ļŲŌ×Ó¼Ū²ćµē×ÓÅŲ¼Ķ¼ĪŖ___________”£
£Ø2£©µŖĖłŌŚÖ÷×åÖŠµŚŅ»µēĄėÄÜ×ī“óµÄŌŖĖŲŹĒ________£ØĢīŌŖĖŲ·ūŗÅ£¬ĻĀĶ¬£©£¬ļŲĖłŌŚÖ÷×åÖŠµēøŗŠŌ×ī“óµÄŌŖĖŲŹĒ____________________”£
£Ø3£©“«Ķ³µÄµŖ»ÆļŲÖʱø·½·ØŹĒ²ÉÓĆGaCl3ÓėNH3ŌŚŅ»¶ØĢõ¼žĻĀ·“Ó¦£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ______________”£
£Ø4£©µŖ»ÆļŲÓė½šøÕŹÆ¾ßÓŠĻąĖĘµÄ¾§Ģå½į¹¹£¬µŖ»ÆļŲÖŠµŖŌ×ÓÓėļŲŌ×ÓÖ®¼äŅŌ____Ļą½įŗĻ£¬µŖ»ÆļŲŹōÓŚ_______¾§Ģ唣
£Ø5£©ĻĀĶ¼ŹĒµŖ»ÆļŲµÄ¾§°ūÄ£ŠĶ£ŗ
¢ŁµŖ»ÆļŲÖŠļŲŌ×ÓµÄŌӻƷ½Ź½ĪŖ__________£¬µŖŌ×ÓµÄÅäĪ»ŹżĪŖ___________”£
¢ŚµŖ»ÆļŲĪŖĮ¢·½¾§°ū£¬µŖ»ÆļŲµÄĆܶČĪŖd g/cm3”£ĮŠ³ö¼ĘĖćµŖ»ÆļŲ¾§°ū±ß³¤aµÄ±ķ“ļŹ½£ŗa=_______cm”£
 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
A”¢B”¢C¶¼ŹĒŌŖĖŲÖÜĘŚ±ķÖŠµÄ¶ĢÖÜĘŚ·Ē½šŹōŌŖĖŲ£¬ĖüĆĒµÄŗĖµēŗÉŹżŅĄ“ĪŌö“ó”£AŌ×ÓµÄŗĖĶā³É¶Ōµē×ÓŹżŹĒĪ“³É¶Ōµē×ÓŹżµÄ2±¶£¬BŌ×ÓµÄ×īĶā²ćp¹ģµĄµÄµē×ÓĪŖ°ėĀś½į¹¹£¬CŹĒµŲæĒÖŠŗ¬Įæ×ī¶ąµÄŌŖĖŲ”£D”¢EŹĒµŚĖÄÖÜĘŚŌŖĖŲ£¬DŌ×ÓŗĖĶā×īĶā²ćµē×ÓŹżÓŠ1øöµē×Ó£¬ĘäÓąø÷²ćµē×Ó¾ł³äĀś£»EŌ×ÓŗĖĶāĪ“³É¶Ōµē×ÓŹżŌŚĶ¬ÖÜĘŚÖŠ×ī¶ą”£ĒėÓƶŌÓ¦µÄŌŖĖŲ·ūŗÅ»ņ»ÆѧŹ½ĢīæÕ£ŗ
£Ø1£©A”¢B”¢CµÄµŚŅ»µēĄėÄÜÓÉŠ”µ½“óµÄĖ³ŠņĪŖ ”£ AŗĶCµÄĒā»ÆĪļ·Šµć“󊔹ŲĻµĪŖ””””””””””””””””£¬ŌŅņĪŖ””””””””””””””””””””””””””””””””””””””””””””””””
””””””””””””””””””””””””””””””””””””””””””””””””””””””””””””””””””””””””””””
£Ø2£©DÓėEµÄŌ×Ó»ÆČČ·Ö±šĪŖ340 kJ”¤mol-1ŗĶ400kJ”¤mol-1£¬ŌņĖüĆĒµÄČŪµć£ŗD E(Ģī”°£¾”±”¢”°£¼”±”¢”°="”±" )”£
£Ø3£©·Ö×ÓA2B2ÖŠ¼üÓė¼üÖ®¼äµÄ¼Š½ĒĪŖ180”ć£¬²¢ÓŠ¶Ō³ĘŠŌ£¬ĪŖ·Ē¼«ŠŌ·Ö×Ó£¬ĆæøöŌ×Ó×īĶā²ćµē×ÓŹż¾łĀś×ć°Ėµē×Ó£¬Ęä½į¹¹Ź½ĪŖ_____________£¬1moløĆ·Ö×ÓÖŠŗ¬ÓŠ ¼üµÄŹżÄæĪŖ ”£
¼üµÄŹżÄæĪŖ ”£
£Ø4£©»łĢ¬£ÅŌ×ÓµÄĶāĪ§µē×ÓÅŲ¼Ź½ĪŖ ”£EO2Cl2ČŪµć£ŗ£96 .5”ę£¬·Šµć£ŗ117”ę£¬Ōņ¹ĢĢ¬EO2Cl2ŹōÓŚ ¾§Ģ唣
£Ø5£©DµÄĒā»ÆĪļµÄ¾§Ģå½į¹¹ČēĶ¼ĖłŹ¾£¬Ęä»ÆѧŹ½ŹĒ ”£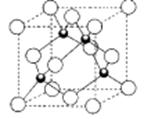
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø14·Ö£©£Ø¢ń£©”¢A”¢B”¢C”¢D”¢E”¢ĪåÖÖŌŖĖŲ¾łŹĒ¶ĢÖÜĘŚŌŖĖŲ£¬ĒŅŌ×ÓŠņŹżŅĄ“ĪŌö“ó”£B”¢EŌ×ÓµÄ×īĶā²ćµē×ÓŹż¾łĪŖĘäµē×Ó²ćŹżµÄ2±¶”£D”¢EŌŖĖŲŌ×ÓµÄ×īĶā²ćµē×ÓŹżĻąµČ”£X”¢Y”¢Z”¢W”¢G”¢¼×”¢ŅŅĘßÖÖĪļÖŹ¾łÓÉÉĻŹöÖŠµÄĮ½ÖÖ»ņČżÖÖŌŖĖŲ×é³É”£ŌŖĖŲBŠĪ³ÉµÄµ„ÖŹMÓė¼×”¢ŅŅ£ØĻą¶Ō·Ö×ÓÖŹĮæ£ŗ¼×<ŅŅ£©ÅØČÜŅŗµÄ·“Ó¦·Ö±šŹĒ£ŗ¼×ÓėM·“Ӧɜ³ÉX”¢Y”¢Z£¬ŅŅÓėM·“Ӧɜ³ÉY”¢Z”¢W£¬·“Ó¦Ģõ¼ž¾łŹ”ĀŌ”£»Ų“šĻĀĮŠÓŠ¹ŲĪŹĢā£ŗ
¢ÅX”¢Y”¢W¾łÄÜÓėZ·“Ó¦£¬Čō½«±ź×¼×“æöĻĀµÄXŗĶD2°“4£ŗ1³äĀśŹŌ¹Üŗó½«Ęäµ¹Į¢ÓŚĖ®²ŪÖŠ£¬“żĖ®²»ŌŚÉĻÉżŹ±£¬ŹŌ¹ÜÄŚČÜÖŹµÄĪļÖŹµÄĮæÅØ¶ČŹĒ £Ø¼ŁÉčČÜÖŹ²»Ą©É¢£©
¢ĘČō½«X”¢W”¢D2°“4£ŗ4£ŗ3ĶØČėZÖŠ³ä·Ö·“Ó¦£¬Š“³ö×ܵĥė×Ó·½³ĢŹ½
¢ĒGŹĒŅ»ÖÖ¼ČÄÜÓėĒæĖįÓÖÄÜÓėĒæ¼ī·“Ó¦µÄĖįŹ½ŃĪ£¬ŌņGµÄµē×ÓŹ½ £¬Č”0.2mol/LµÄNaOHČÜŅŗÓė0.1mol/LµÄGČÜŅŗµČĢå»ż»ģŗĻŗ󣬼ÓČČÖĮ³ä·Ö·“Ó¦ŗ󣬓ż»Öø“ÖĮŹŅĪĀŹ£ÓąČÜŅŗÖŠĄė×ÓÅØ¶ČµÄÓɓ󵽊”Ė³ŠņŹĒ £¬“ĖŹ±²āµĆČÜŅŗµÄPH=12£¬Ōņ“ĖĢõ¼žĻĀGÖŠŅõĄė×ӵĵēĄėĘ½ŗā³£ŹżKa =
£Ø¢ņ£©¢ČijĪĀ¶ČŹ±£¬ĻņAgNO3ČÜŅŗÖŠ¼ÓČėK2CrO4ČÜŅŗ»įÉś³É
Ag2CrO4³Įµķ£¬Ag2CrO4ŌŚĖ®ÖŠµÄ³ĮµķČܽāĘ½ŗāĒśĻßČēĶ¼ĖłŹ¾”£
øĆĪĀ¶ČĻĀ£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ_________”£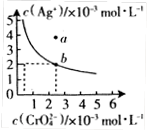
A. Ag2CrO4µÄČܶȻż³£Źż(Ksp)ĪŖ1”Į10£8
B. ŗ¬ÓŠ“óĮæCrO µÄČÜŅŗÖŠŅ»¶Ø²»“ęŌŚAg+
µÄČÜŅŗÖŠŅ»¶Ø²»“ęŌŚAg+
C. aµć±ķŹ¾Ag2CrO4µÄ²»±„ŗĶČÜŅŗ£¬Õō·¢æÉŅŌŹ¹ČÜŅŗÓÉaµć±äµ½bµć
D. 0.02mol”¤L£1µÄAgNO3ČÜŅŗÓė0.02mol”¤L£1µÄNa2CrO4ČÜŅŗµČĢå»ż»ģŗĻ»įÉś³É³Įµķ
¢ÉČō³£ĪĀĻĀKsp[Cr(OH)3]=10£32£¬ŅŖŹ¹c(Cr3+)½µÖĮ10£5mol”¤L£1£¬ČÜŅŗµÄpHÓ¦µ÷ÖĮ_______”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø12·Ö£©A”¢B”¢C”¢DĖÄÖÖŌŖĖŲ£¬Ō×ÓŠņŹżŅĄ“ĪŌö“ó£¬AŌ×ÓµÄ×īĶā²ćÉĻÓŠ4øöµē×Ó£»BµÄŅõĄė×ÓŗĶCµÄŃōĄė×Ó¾ßÓŠĻąĶ¬µÄµē×Ó²ć½į¹¹£¬Į½ŌŖĖŲµÄµ„ÖŹ·“Ó¦£¬Éś³ÉŅ»ÖÖµ»ĘÉ«µÄ¹ĢĢåE£¬DµÄL²ćµē×ÓŹżµČÓŚK”¢MĮ½øöµē×Ó²ćÉĻµÄµē×ÓŹżÖ®ŗĶ”£
£Ø1£©DŌŖĖŲŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĪ»ÖĆ £¬øĆŌŖĖŲ³£¼ūŃõ»ÆĪļÓŠ ŗĶ £ØŠ“³ö»ÆѧŹ½£©
£Ø2£©Š“³öB”¢Cµ„ÖŹÉś³É£ÅµÄ»Æѧ·½³ĢŹ½£ŗ”” ”””””””””””””””””£
£Ø3£©12æĖ£Įµ„ÖŹ³ä·ÖČ¼ÉÕŗóµÄ²śĪļĶØČĖ1L 1mol/LNaOHČÜŅŗÖŠ£¬ĖłµĆČÜŅŗµÄČÜÖŹĪŖ ,ĘäĪļÖŹµÄĮæĪŖ mol”£
£Ø4£©Š“³öDµÄ×īøß¼ŪŃõ»ÆĪļµÄĖ®»ÆĪļŗĶA·“Ó¦µÄ»Æѧ·½³ĢŹ½””””””””””””””””””””””
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ķ¼±ķ·Ø”¢Ķ¼Ļń·ØŹĒ³£ÓƵÄæĘѧъ¾æ·½·Ø”£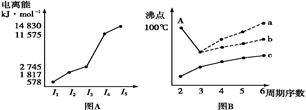
¢ń.Ķ¼(A)ŹĒ¶ĢÖÜĘŚÄ³Ö÷×åŌŖĖŲXµÄµēĄėÄÜĖłŹ¾Ēéæö”£ŌņXŌŖĖŲĪ»ÓŚÖÜĘŚ±ķµÄµŚ ×唣
Ķ¼BŹĒŃŠ¾æ²æ·ÖŌŖĖŲµÄĒā»ÆĪļµÄ·Šµć±ä»Æ¹ęĀɵÄĶ¼Ļń,ÕŪĻßcæÉŅŌ±ķ“ļ³öµŚ ×åŌŖĖŲĒā»ÆĪļµÄ·ŠµćµÄ±ä»Æ¹ęĀÉ”£
¢ņ.ĻĀ±ķŹĒŌŖĖŲÖÜĘŚ±ķµÄŅ»²æ·Ö,±ķÖŠĖłĮŠµÄ×ÖÄø·Ö±š“ś±ķŅ»ÖÖ»ÆѧŌŖĖŲ”£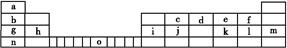
ŹŌ»Ų“šĻĀĮŠĪŹĢā:
(1)ĒėŠ“³öŌŖĖŲoµÄĶāĪ§µē×ÓÅŲ¼Ź½: ”£
(2)ÓÉjŌ×ÓøścŌ×ÓŅŌ1”Ć1Ļą»„½»Ģę½įŗĻ¶ųŠĪ³ÉµÄ¾§Ģå,¾§ĢåĄąŠĶÓė¾§ĢåjĻąĶ¬”£Į½ÕßĻą±ČČŪµćøüøߵďĒ (Ģī»ÆѧŹ½),ŹŌ“Ó½į¹¹½Ē¶Č¼ÓŅŌ½āŹĶ: ”£
(3)iµ„ÖŹ¾§ĢåÖŠŌ×ӵĶѻż·½Ź½ČēĻĀĶ¼¼×ĖłŹ¾,Ę侧°ūĢŲÕ÷ČēĻĀĶ¼ŅŅĖłŹ¾,Ō×ÓÖ®¼äĻą»„Ī»ÖĆ¹ŲĻµµÄĘ½ĆęĶ¼ČēĻĀĶ¼±ūĖłŹ¾”£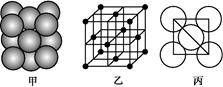
Ēė»Ų“š:¾§°ūÖŠiŌ×ÓµÄÅäĪ»ŹżĪŖ ,Ņ»øö¾§°ūÖŠiŌ×ӵďżÄæĪŖ £¬øĆ¾§°ūµÄæÕ¼äĄūÓĆĀŹĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¶ĢÖÜĘŚŌŖĖŲA”¢B”¢C”¢D”¢EµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó£¬AŌŖĖŲŃōĄė×ÓµÄŌ×ÓŗĖĶāƻӊµē×Ó£¬BŹĒæÕĘųÖŠŗ¬Įæ×ī¶ąµÄŌŖĖŲ£»CŌŖĖŲŌ×Ó×īĶā²ćµē×ÓŹżŹĒĘäµē×Ó²ćŹżµÄČż±¶£»CÓėDæÉŠĪ³ÉĮ½ÖÖ³£¼ūµÄĄė×Ó»ÆŗĻĪļ£»¹¤ŅµÉĻ³£ÓƵē½āCÓėEµÄ»ÆŗĻĪļĄ“ÖʱøEµ„ÖŹ”£
£Ø1£©»³öDŌŖĖŲµÄŌ×Ó½į¹¹Ź¾ŅāĶ¼ ”£C”¢D”¢EµÄ¼ņµ„Ąė×Ó°ė¾¶ÓÉŠ”µ½“óµÄĖ³Šņ £ØÓĆĄė×Ó·ūŗűķŹ¾£©”£
£Ø2£©Š“³öŌŖĖŲEŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆ ”£
£Ø3£©¹¤ŅµÉĻ³£ÓĆAŗĶBµÄµ„ÖŹŗĻ³ÉŅ»ÖÖ³£¼ūĘųĢ壬¼ģŃéøĆĘųĢåµÄ³£ÓĆ·½·ØŹĒ ”£
£Ø4£©D2C2ÓėH2O·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ £¬D2C2ÓėCuSO4ČÜŅŗ·“Ó¦µÄĻÖĻóŹĒ ”£
£Ø5£©ÓÉŌŖĖŲA”¢B”¢C°“Ō×ÓøöŹż±Č4£ŗ2£ŗ3ŠĪ³ÉµÄ»ÆŗĻĪļ£¬Š“³öĘäµēĄė·½³ĢŹ½£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĻĀ±ķĪŖŌŖĖŲÖÜĘŚ±ķµÄŅ»²æ·Ö£¬±ķÖŠĮŠ³öĮĖ11ÖÖŌŖĖŲŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆ£¬°“ŅŖĒóĶź³ÉĻĀĮŠø÷Š”Ģā”£
| Ö÷×å ÖÜĘŚ | ¢ńA | ¢ņA | ¢óA | ¢ōA | ¢õA | ¢öA | ¢÷A | 0 |
| 2 | | | | ¢Ž | | ¢ß | ¢į | |
| 3 | ¢Ł | ¢Ū | ¢Ż | | | | ¢ą | ¢ā |
| 4 | ¢Ś | ¢Ü | | | | | | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĻĀ±ķĪŖŌŖĖŲÖÜĘŚ±ķµÄŅ»²æ·Ö£¬Ēė²ĪÕÕŌŖĖŲ¢Ł”«¢įŌŚ±ķÖŠµÄĪ»ÖĆ£¬ÓĆ»ÆѧÓĆÓļ»Ų“šĻĀĮŠĪŹĢā£ŗ
| Ö÷×å ÖÜĘŚ | ¢ńA | ¢ņA | ¢óA | ¢ōA | ¢õA | ¢öA | ¢÷A | 0 |
| Ņ» | ¢Ł | | | | | | | |
| ¶ž | | | | ¢Ś | | ¢Ū | ¢Ü | |
| Čż | ¢Ż | | ¢Ž | ¢ß | ¢ą | | ¢į | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÓŠA”¢B”¢C”¢D”¢EĪåÖÖ¶ĢÖÜĘŚŌŖĖŲ,ŅŃÖŖĻąĮŚµÄA”¢B”¢C”¢DĖÄÖÖŌŖĖŲŌ×ÓŗĖĶā¹²ÓŠ56øöµē×Ó,ŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆČēĶ¼ĖłŹ¾”£EµÄµ„ÖŹæÉÓėĖį·“Ó¦,1 mol Eµ„ÖŹÓė×ćĮæĖį×÷ÓĆ,ŌŚ±ź×¼×“æöĻĀÄܲśÉś33.6 L H2;EµÄŃōĄė×ÓÓėAµÄŅõĄė×ÓŗĖĶāµē×Ó²ć½į¹¹ĶźČ«ĻąĶ¬”£
»Ų“šĻĀĮŠĪŹĢā: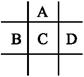
(1)AÓėEŠĪ³ÉµÄ»ÆŗĻĪļµÄ»ÆѧŹ½ŹĒ”””””””””£
(2)BµÄ×īøß¼ŪŃõ»ÆĪļ»ÆѧŹ½ĪŖ””””””””,CµÄŌŖĖŲĆū³ĘĪŖ””””””””,DµÄµ„ÖŹÓėĖ®·“Ó¦µÄ·½³ĢŹ½ĪŖ””____________________________
(3)ĻņDÓėEŠĪ³ÉµÄ»ÆŗĻĪļµÄĖ®ČÜŅŗÖŠµĪČėÉÕ¼īČÜŅŗÖ±ÖĮ¹żĮæ,¹Ū²ģµ½µÄĻÖĻóŹĒ
_____________________________________________________
ÓŠ¹Ų·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ___________________________
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com