
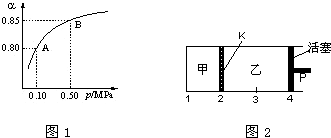
���� ��1���ٲ��ù�����O2��������߶�������ת���ʣ�
�ڽ�0.050molSO2��g����0.030molO2��g�������ݻ�Ϊ1L���ܱ������У���һ�������´ﵽƽ�⣬���c��SO3��=0.040mol/L����
2SO2��g��+O2��g��?2SO3��g��
��ʼŨ�ȣ�mol/L����0.05 0.03 0
�仯Ũ�ȣ�mol/L����0.04 0.02 0.04
ƽ��Ũ�ȣ�mol/L����0.01 0.01 0.04
�ٸ���K=$\frac{{c}^{2}��S{O}_{3}��}{{c}^{2}��S{O}_{2}����c��{O}_{2}��}$����ƽ�ⳣ����
����֪��K��300�棩��K��350�棩�������¶�ƽ�ⳣ����С��˵��ƽ�������ƶ����������¶�ƽ�������ȷ�Ӧ�����ƶ���
��2��ƽ�ⳣ��ֻ���¶�Ӱ�죬�¶Ȳ��䣬ƽ�ⳣ�����䣻
��3���ټ�Ϊ���º������������ƶ�����P��ʹ�ҵ��ݻ��ͼ���ȣ�Ϊ���º������������м���4mol���������൱�ڼ���2mol���������ټ���2mol������������ѹǿ��ƽ��������У�SO3�������������
�ڼ�Ϊ���º������������뺤����ѹ����ѹ���䣬ƽ�ⲻ�䣬���м��뺤��Ϊ���ֺ�ѹ���������ѹǿ��С��ƽ��������У��ﵽ��ƽ��ʱ��SO3�����������С��
��� �⣺��1���ٴ�ƽ��Ƕȷ������ù���O2��Ŀ���ǣ���������ԭ���������ת���ʣ�����������߶��������ת���ʣ�
�ʴ�Ϊ����߶��������ת���ʣ�
�ڽ�0.050molSO2��g����0.030molO2��g�������ݻ�Ϊ1L���ܱ������У���һ�������´ﵽƽ�⣬���c��SO3��=0.040mol/L����
2SO2��g��+O2��g��?2SO3��g��
��ʼŨ�ȣ�mol/L����0.05 0.03 0
�仯Ũ�ȣ�mol/L����0.04 0.02 0.04
ƽ��Ũ�ȣ�mol/L����0.01 0.01 0.04
ƽ�ⳣ��K=$\frac{{c}^{2}��S{O}_{3}��}{{c}^{2}��S{O}_{2}����c��{O}_{2}��}$=$\frac{0.0{4}^{2}}{0.0{1}^{2}��0.01}$=1600��
�ʴ�Ϊ��1600��
��K��300�棩��K��350�棩��˵���¶�Խ��ƽ�ⳣ��ԽС����Ӧ������У�����ƽ�������ȷ�Ӧ������У�����ӦΪ���ȷ�Ӧ������ƽ��������У���������ת���ʼ�С��
�ʴ�Ϊ���ţ���С��
��2��ƽ�ⳣ��ֻ���¶�Ӱ�죬��ѹǿ�أ�ƽ��״̬��A�䵽Bʱ�������¶���ͬ����ƽ�ⳣ��K��A��=K��B�����ʴ�Ϊ��=��
��3����2mol SO2��1mol O2����������У���4mol SO3�����������У�����K�����ƶ�����ʱ���ƻ���P��ʹ�ҵ��ݻ�Ϊ��2������֪�ס��������ﵽ��ͬ��ƽ��״̬��
�����ƶ�����P��ʹ�ҵ��ݻ��ͼ���ȣ�����ѹǿ��ƽ��������У�SO3�������������SO3����������ף��ң�
�ʴ�Ϊ������
�ڼ�Ϊ���º������������뺤����ѹ����ѹ���䣬ƽ�ⲻ�䣬���м��뺤����Ϊ���ֺ�ѹ���������ѹǿ��С��ƽ��������У��ﵽ��ƽ��ʱ��SO3�����������С��SO3����������״����ң�
�ʴ�Ϊ������
���� ���⿼�黯ѧƽ�ⳣ������ѧƽ�������Ӱ�����أ���Ŀ�Ѷ��еȣ�ע��ƽ�ⳣ���ڼ����е�Ӧ�ã���3���йؼ��ǹ�����Чƽ�⽨��;����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��Ļ�ѧʽ ���볣��K | ������ H2S | ������ H2SO3 | ���� H2CrO4 | ���� HCN |
| K1 | 9.1��10-8 | 1.5��10-2 | 1.8��10-1 | 5.0��10-10 |
| K2 | 1.1��10-12 | 1.0��10-7 | 3.2��10-7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 CO2��CO�ǹ�ҵ�ŷŵĶԻ�������Ӱ��ķ�����
CO2��CO�ǹ�ҵ�ŷŵĶԻ�������Ӱ��ķ�����| ���� | CH3OH | CH3OCH3 | H2O |
| Ũ��/��mol•L-1�� | 0.01 | 0.2 | 0.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʱ��/��min�� | 2 | 4 | 6 | 8 |
| n��SO3��/��mol�� | 4.2 | 8.0 | 9.4 | 9.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CaCO3 | B�� | NaHCO3 | C�� | BaCO3 | D�� | Na2CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  | B�� |  | ||
| C�� | CH2�TCHC��CH3��2CH��CH3��2 | D�� | ��CH3��3CC��CH3���TCHCH3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com