
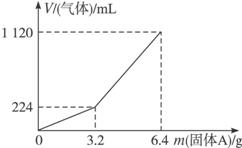
��1��3.2 g����A��������������__________�������ʵ����ʵ����ֱ�Ϊ__________���������������ַ�Ӧ��������Һ�����ʵ����ʵ���Ũ��Ϊ��������Һ����仯��__________��
��2��������Aȫ�������������ᣬ��A������m��3.2 gʱ���ռ����������������״���£�V��__________mL���ú�m�Ĵ���ʽ��ʾ����
��1��Fe��FeS n(Fe)��0.01 mol��n(FeS)��0.03 mol c(H2S)��0.1 mol��L-1��c(FeCl2)��0.13 mol��L-1 ��2��(280m��672)
�������������������л�ѧ��ӦΪ�����������Թ�ϵʽΪ�������ۺϼ��㣬�漰�غ㷨�ͼ�ֵ����ͬʱ��������ѧ����ʶͼ������������ѧ֪ʶ�����ѧ�����������
Fe��S��������ʱ���ȣ�������FeS�⣬��������Fe��S��������������뵽300 mL 2 mol��L-1 HCl��ʱ���ܹ���ȫ�ܽ⣬��˵����������S����HCl������������Ŀ������֪��H2��H2S���ɣ����ȷ������AΪFeS����Fe�Ļ�������ͼ������֪����A������m��3.2 gʱ���ռ���������ֻ��H2����ʱH2S����ˮ�У���m=3.2 gʱ��H2S��ˮ�е��ܽ�ﵽ���͡���m��3.2 gʱ����H2Sˮ��Һ�Ѿ����ͣ���ʱ�����H2S����������������Ϊ����1������ͼ֪�ڹ���A������m=3.2 gʱ��H2S��ˮ�е��ܽ�ﵽ���ͣ���ʱ�ռ�����������ֻ��H2��
n(H2)��0.224 L/22.4 L��mol-1 ��0.01 mol
��m(Fe)��0.01 mol��56 g��mol-1��0.56 g
m(FeS)��3.2 g��0.56 g��2.64 g
��n(FeS)��2.64 g/88 g��mol-1��0.03 mol
c(H2S)��n(H2S)/V(Һ)��0.03 mol/0.3 L��0.1 mol��L-1
c(FeCl2)��n(Fe)/V(Һ)=��0.01 mol+0.03 mol��/0.3 L��0.13 mol��L-1
��2����m��3.2 gʱ���ɹ�ϵʽ��Fe��H2��FeS��H2S�������غ�֪��
����A�Ͳ�����������ʵ���֮��Ϊ1��1����m g����ɲ���H2��H2S�����Ϊ��m/3.2��896��280m��mL����������672 mL��H2S�ܽ�����Һ�У����ԣ�ʵ���ռ���������Ϊ����280m��672��mL��


 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ����һ��2010��������Ĵ��¿�����ѧ�Ծ� ���ͣ�022
��һ�����Ļ�Ͼ��ȵ����ۺ�����ڸ��������������¹��ȣ���ַ�Ӧ����ȴ�����£��õ�����A��������Ϊm�Ĺ���A���뵽300 mL��2 mol��L��1������ʹ֮��ȫ�ܽ⣬��������¼���Ĺ���A���������ռ������������(�ѻ���ɱ�״��)�Ĺ�ϵ��ͼ��(������������Һ����֮ǰ�������ݳ�)����֪�������A������m��3.2 gʱ���ռ���������Ϊ��������m��3.2 gʱ�ռ���������Ϊ����������Ļ������
�Է�������㣺
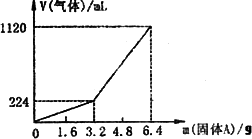
(1)3.2 g����A��������������(д��ѧʽ)________��
(2)3.2 g����A�и����ʵ����ʵ����ֱ�Ϊ________���������������ַ�Ӧ�����õ���Һ������������ʵ���Ũ��Ϊ(��������仯)________��
(3)������Aȫ�������������ᣬ��A������m��3.2 gʱ���ռ������������(��״����)V��________mL��(�ú�m�Ĵ���ʽ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��֪�������A������m��3.2 gʱ���ռ���������Ϊ��������m��3.2 gʱ���ռ���������ΪH2��H2S�Ļ�����塣
�Է������㣺
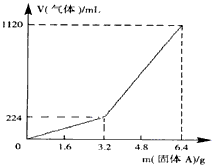
��1��3.2 g����A��������������_____________��
��2��3.2 g����A�и����ʵ����ʵ����ֱ�Ϊ_______________���������������ַ�Ӧ��������Һ������������ʵ���Ũ��Ϊ��������Һ����ı仯��______________��
��3��������Aȫ�������������ᣬ��A������m��3.2 gʱ���ռ�������������������V=____________mL(�ú�m�Ĵ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0103 �¿��� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ������Ͼ��ȵ����ۺ���۸����������ȣ���ַ�Ӧ����ȴ�����µõ�����A��������Ϊa�Ĺ���A���뵽 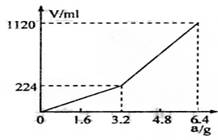 300mL2mol?L��1������ʹ֮��ȫ�ܽ⡣�ռ�����������V����״���²ⶨ����������A������a֮��Ĺ�ϵ��ͼ��ʾ��������������������Һ����ǰ�����������ݳ�������֪�������A������a��3.2gʱ���ռ���������Ϊ��������a>3.2g���ռ���������ΪH2��H2S�Ļ�������Է�������㣺
300mL2mol?L��1������ʹ֮��ȫ�ܽ⡣�ռ�����������V����״���²ⶨ����������A������a֮��Ĺ�ϵ��ͼ��ʾ��������������������Һ����ǰ�����������ݳ�������֪�������A������a��3.2gʱ���ռ���������Ϊ��������a>3.2g���ռ���������ΪH2��H2S�Ļ�������Է�������㣺
��1��3.2g����A��������������_________________________________
��2��3.2g����A�и����ʵ����ʵ����ֱ�Ϊ________________���������������ַ�Ӧ��������Һ������������ʵ���Ũ��Ϊ��������Һ����ı仯�� ��
��3��������Aȫ�������������ᣬ��A������a>3.2gʱ���ռ����������������״���£�V=__________________________mL���ú�a�Ĵ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com