
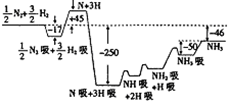
 2007���ŵ������ѧ������߸����•���ض������˺ϳɰ���Ӧ������T=673Kʱ��������Ӧ�������仯��ͼ��ʾ��ͼ�е�������λΪkJ•mol-1����ע��ͼ�С�������ʾ�ڴ����������������ش��������⣺
2007���ŵ������ѧ������߸����•���ض������˺ϳɰ���Ӧ������T=673Kʱ��������Ӧ�������仯��ͼ��ʾ��ͼ�е�������λΪkJ•mol-1����ע��ͼ�С�������ʾ�ڴ����������������ش��������⣺���� ��1������ͼ�����еĻ��������ƶϳ��١���������Ӧ��
��2����ͼ���Կ�������1molNH3����H=-46kJ•mol-1������������ı�ķ�Ӧ����һ������������У�ѹǿ���ֲ�����ƽ��״̬��
��3����ƽ��״̬���淴Ӧ������ȣ�����ֵ�Ũ�ȱ��ֲ��䣬�ɴ˷������
��4������ˮ�����ӻ�������ȿɼ���pNH4��
��5������N2H4�����뵽NH3����N2H4Cl2���뵽NH4Cl����ע�N2H4�Ƕ�Ԫ���NH3��һԪ�ģ����⼴��ͻ�ƣ�
��a����������ǿ�������Σ���ˮ�⣾ˮ��������ӣ��������ӣ��������ӣ�����c��Cl-����c��N2H62+����c��H+����c��OH-������
b��ˮ������c��[N2H5•H2O]+��=c��H+�������ܼ�ˮҲ������������ӣ�����c��Cl-����c��H+����c��[N2H5•H2O]+����c��OH-����
c���������е���غ�2c��N2H62+��+c��[N2H5•H2O]+��+c��H+��=c��OH-��+c��Cl-����
d����������ǿ�������Σ���ˮ�⣾ˮ��������ӣ��������ӣ��������ӣ�����c��Cl-����c��N2H62+����c��H+����c��OH-�����ɴ˷������
��� �⣺��1����ͼ��֪�÷�Ӧ�Ļ�����H2��H2����2H��2H����N2����2N��2N����N��+H����NH����NH2����NH3����NH3��
��ٵķ�ӦΪH2?H2��?2H?2H����
�۵ķ�ӦΪN��+H��?NH����
�ʴ�Ϊ����H2?H2��?2H?2H������N��+H��?NH����
��2����ͼ���Կ�������1molNH3����H=-46kJ•mol-1����Ӧ���Ȼ�ѧ����ʽΪN2��g��+3H2��g��?2NH3��g������H=-92kJ•mol-1��
�ʴ�Ϊ��N2��g��+3H2��g��?2NH3��g������H=-92kJ•mol-1��
��3��a.3v����N2��=v����H2��=v�棨H2������ƽ��״̬������ȷ��
b.3v����N2��=v����H2��������Ӧ������Ӧ����δ����������Ĺ�ϵ���ʴ���
c��������ѹǿ���ֲ��䣬˵����������ʵ������䣬��Ӧ��ƽ��״̬������ȷ��
d�����������ܶ�ʼ�ձ��ֲ��䣬����˵����ƽ��״̬���ʴ���ѡac��
��4���������֪c��NH4+����c��NH2-��=10-30��c��NH4+��=$\sqrt{1{0}^{-30}}$mol/L=10-15mol/L��pNH4=15��
�ʴ�Ϊ��15��
��5������N2H4�����뵽NH3����N2H6Cl2���뵽NH4Cl����ע�N2H4�Ƕ�Ԫ���NH3��һԪ�ģ��������µ�һ��ˮ�ⷴӦ�����ӷ���ʽΪN2H62++H2O?[N2H5•H2O]++H+��
�ʴ�Ϊ��N2H62++H2O?[N2H5•H2O]++H+��
��a����������ǿ�������Σ���ˮ�⣾ˮ��������ӣ��������ӣ��������ӣ�����c��Cl-����c��N2H62+����c��H+����c��OH-������a��ȷ����
b��ˮ������c��[N2H5•H2O]+��=c��H+�������ܼ�ˮҲ������������ӣ�����c��Cl-����c��H+����c��[N2H5•H2O]+����c��OH-������b����
c���������е���غ�2c��N2H62+��+c��[N2H5•H2O]+��+c��H+��=c��OH-��+c��Cl-������c��ȷ��
d����������ǿ�������Σ���ˮ�⣾ˮ��������ӣ��������ӣ��������ӣ�����c��Cl-����c��N2H62+����c��H+����c��OH-������d����
��ѡac��
���� ����ܺõĽ���ѧƽ�⡢����ƽ�⡢��Ӧ�Ƚ����һ�飬��Ȼ��Ŀ������ŵ���������йر���������������㣬�������ⷽ���Ǹ߿��ij��÷�����һ��Ҫ��������Ƿ�������Ϣ��������Щ�Ǹ�����Ϣ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| Ԫ�� | A | B | C |
| �ṹ��Ϣ | ��̬ԭ�Ӻ������������Ӳ㣬�������3��δ�ɶԵĵ��� | ��̬ԭ�ӵ�M����1�ԳɶԵ�p���� | ��̬ԭ�Ӻ���M��ȫ��������N����һ��δ�ɶԵ��� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2SO4��Ħ������Ϊ98g | |
| B�� | 1molH2SO4������Ϊ98g/mol | |
| C�� | ��״����2molO2�����ԼΪ44.8L | |
| D�� | ���³�ѹ������Ħ�����ԼΪ22.4L/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 3A+B=C | B�� | 2A+2B=3C | C�� | 4A+6B=9C | D�� | 9A+6B=4C |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 �о���Ա������һ�֡�ˮ������أ����ܷ�ӦΪ��5Mn02+2Ag+2NaCl=Na2Mn5O10+2AgCl����ͼ�á�ˮ�����Ϊ��Դ���NaCl��Һ��ʵ���У�X�缫������ɫ�����ݳ��������йط�����ȷ���ǣ�������
�о���Ա������һ�֡�ˮ������أ����ܷ�ӦΪ��5Mn02+2Ag+2NaCl=Na2Mn5O10+2AgCl����ͼ�á�ˮ�����Ϊ��Դ���NaCl��Һ��ʵ���У�X�缫������ɫ�����ݳ��������йط�����ȷ���ǣ�������| A�� | IΪ��������缫��ӦʽΪAg+Cl--e-=AgCl | |
| B�� | ��ˮ�������Na+�������������ƶ� | |
| C�� | ÿת��1mole-��U��������0.5mol H2O | |
| D�� | ��ʼʱU����Y������pH������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
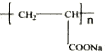
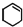 ��Ϊԭ�Ϻϳɣ�·�����£����ַ�Ӧ����ʡ�ԣ���
��Ϊԭ�Ϻϳɣ�·�����£����ַ�Ӧ����ʡ�ԣ���
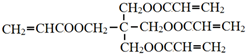
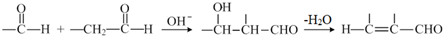

 �Ļ�ѧ����ʽ��
�Ļ�ѧ����ʽ�� ��
��
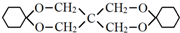 ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ɫ��ѧ�ĺ��ľ���������ҵ�����Ի�����������Ⱦ | |
| B�� | �����ơ��ⶼ������ز����ٵ���Ԫ�� | |
| C�� | ʳ�ο�����ζ������������ʳƷ������ | |
| D�� | ���ع��͡������ӹ��������������Ʒ������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com