
| AЃЎC(s) +1/2O2(g) ="=CO(g);" ЁїHЃН-110.5 kJ/mol |
| BЃЎCO(g) +1/2O2(g) ==CO2(g); ЁїHЃН-283.0 kJ/mol |
| CЃЎH2(g) +1/2O2(g)==H2O(g); ЁїHЃН-241.8 kJ/mol |
| DЃЎ2C8H18(l) +25O2(g)==16CO2(g)+18H2O(l); ЁїHЃН-11036 kJ/mol |
 жЅТщПЊЛЈПЮГЬаТЬхбщЯЕСаД№АИ
жЅТщПЊЛЈПЮГЬаТЬхбщЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЕЅбЁЬт
| AЃЎ1 264 kJЁЄmolЃ1 | BЃЎ632 kJЁЄmolЃ1 | CЃЎ316 kJЁЄmolЃ1 | DЃЎ1 624 kJЁЄmolЃ1 |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЬюПеЬт
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЕЅбЁЬт
| AЃЎ+882kJЁЄmol-1 | BЃЎ+441kJЁЄmol-1 |
| CЃЎЃ882kJЁЄmol-1 | DЃЎЃ441kJЁЄmol-1 |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЕЅбЁЬт

| AЃЎN2(g)ЃЋ3H2(g)===2NH3(l) ІЄHЃН2(aЃbЃc)kJЁЄmolЃ |
| BЃЎN2(g)ЃЋ3H2(g)===2NH3(g) ІЄHЃН2(bЃa)kJЁЄmolЃ1 |
| CЃЎ1/2 N2 (g)ЃЋ3/2H2(g)===NH3(l) ІЄHЃН(bЃЋcЃa)kJЁЄmolЃ1 |
| DЃЎ1/2 N2(g)ЃЋ3/2H2(g)===NH3(g) ІЄHЃН(aЃЋb)kJЁЄmolЃ1 |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЕЅбЁЬт
| ЛЏбЇМќ | HЁЊH | ClЁЊCl | HЁЊCl |
| ЩњГЩЃЈВ№ПЊЃЉ1molЛЏбЇМќЗХГіЃЈЮќЪеЃЉЕФФмСП | 436kJ | 243kJ | 431kJ |
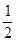 H2(g)+
H2(g)+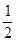 Cl2(g)="=HCl(g)" ЃЛЁїHЃНЃ91.5kJЁЄmolЃ1
Cl2(g)="=HCl(g)" ЃЛЁїHЃНЃ91.5kJЁЄmolЃ1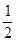 H2(g)+
H2(g)+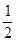 Cl2(g)="=HCl(g)" ЃЛЁїHЃН+91.5kJЁЄmolЃ1
Cl2(g)="=HCl(g)" ЃЛЁїHЃН+91.5kJЁЄmolЃ1ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЬюПеЬт
 2NH3ЃЈgЃЉ +
2NH3ЃЈgЃЉ + 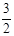 O2ЃЈgЃЉ ЃЛЁїH =" a" kJЁЄmolЁЊ1
O2ЃЈgЃЉ ЃЛЁїH =" a" kJЁЄmolЁЊ1| T/K | 303 | 313 | 323 |
| NH3ЩњГЩСП/ЃЈ10-6molЃЉ | 4ЃЎ8 | 5ЃЎ9 | 6ЃЎ0 |
 2NH3ЃЈgЃЉ ІЄH= Ѓ92ЃЎ4kJЁЄmolЁЊ1
2NH3ЃЈgЃЉ ІЄH= Ѓ92ЃЎ4kJЁЄmolЁЊ1 2H2OЃЈlЃЉ ІЄH = Ѓ571ЃЎ6kJЁЄmolЁЊ1
2H2OЃЈlЃЉ ІЄH = Ѓ571ЃЎ6kJЁЄmolЁЊ1ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЕЅбЁЬт
| AЃЎaЃОbЃОc | BЃЎaЃОcЃОb | CЃЎcЃОbЃОa | DЃЎbЃОaЃОc |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЕЅбЁЬт
| AЃЎЫсФмЕчРыГіH+КЭЫсИљРызгЃЌЙЪЫсЮЊРызгЛЏКЯЮя |
| BЃЎЙВМлЛЏКЯЮяЗжзгжавЛЖЈКЌгаЙВМлМќ |
| CЃЎЧтбѕЛЏБЕОЇЬхгыТШЛЏяЇЙЬЬхЕФЗДгІЪЧЮќЪеШШСПЕФЗДгІ |
| DЃЎашМгШШЕФЗДгІВЛвЛЖЈЪЧЮќШШЗДгІ |
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com