
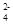 ֊єȔŹŹµ±µÄĄė×Ó×é³É·ūŗĻĻĀĮŠĒéæöµÄµē½āÖŹ£¬½ųŠŠµē½ā(ŃōĄė×ÓÖ»ÄÜŹ¹ÓĆŅ»“Ī)”£
֊єȔŹŹµ±µÄĄė×Ó×é³É·ūŗĻĻĀĮŠĒéæöµÄµē½āÖŹ£¬½ųŠŠµē½ā(ŃōĄė×ÓÖ»ÄÜŹ¹ÓĆŅ»“Ī)”£ H2”ü£«Cl2”ü
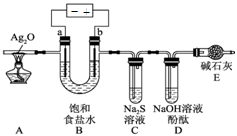
H2”ü£«Cl2”ü 2Cu£«O2”ü£«2H2SO4
2Cu£«O2”ü£«2H2SO4 H2”ü£«Cl2”ü”£
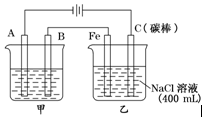
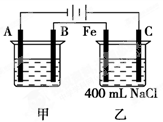
H2”ü£«Cl2”ü”£ 2Cu£«O2”ü£«2H2SO4”£
2Cu£«O2”ü£«2H2SO4”£

 ŗ®¼Ł““ŠĀŠĶ×ŌÖ÷ѧĻ°µŚČżŃ§ĘŚŗ®¼ŁĻĪ½ÓĻµĮŠ“š°ø
ŗ®¼Ł““ŠĀŠĶ×ŌÖ÷ѧĻ°µŚČżŃ§ĘŚŗ®¼ŁĻĪ½ÓĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®CuSO4 | B£®Cu(OH)2 | C£®Cu | D£®CuO |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®2£®7 mol”¤ L-1 | B£®3 mol”¤ L-1 | C£®4 mol”¤ L-1 | D£®1 mol”¤ L |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
 Cd + 2NiO(OH)+ 2H2O
Cd + 2NiO(OH)+ 2H2O | A£®Cd | B£®NiO(OH) | C£®Cd(OH)2 | D£®Ni(OH)2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com