
 Fe(OH)3(����)+3H+ ��1�֣�
Fe(OH)3(����)+3H+ ��1�֣�  2 C2H5OH + O2
2 C2H5OH + O2  2 CH3CHO + 2H2O����2�֣�
2 CH3CHO + 2H2O����2�֣� Fe(OH)3(����)+3H+��H+ȫ������ˮ�������ɵģ�pH=2��c(H+)=10-2mol/L��c(OH-)ˮ= c(H+)ˮ=10-2mol/L���������������ӣ��ﵽ��ˮ��Ŀ�ġ���2����NaHSO4��Һ�е�H+����ˮ�ĵ����NaHSO4��ˮ�еĵ��룬 OH-����ˮ�ĵ��룬c(OH-)ˮ������ˮ�����c(H+)ˮ��c(SO42-)������NaHSO4��ˮ�еĵ����H+��c(H+)�����c(H+)=c(OH-)��c(SO42-)������Һ��SO42-��ȫ������NaHSO4+Ba(OH)2= BaSO4��+H2O+NaOH����Ӧ������ΪNaOH���ʼ��ԣ�pH>7����3��������ԭBaCl2��Һ����СŨ��Ϊx,Ksp(BaSO4)= c(Ba2+)* c(SO42-),1.1��10-10=(0.02/2)*(x/2),���x=2.2��10-8mol/L��
Fe(OH)3(����)+3H+��H+ȫ������ˮ�������ɵģ�pH=2��c(H+)=10-2mol/L��c(OH-)ˮ= c(H+)ˮ=10-2mol/L���������������ӣ��ﵽ��ˮ��Ŀ�ġ���2����NaHSO4��Һ�е�H+����ˮ�ĵ����NaHSO4��ˮ�еĵ��룬 OH-����ˮ�ĵ��룬c(OH-)ˮ������ˮ�����c(H+)ˮ��c(SO42-)������NaHSO4��ˮ�еĵ����H+��c(H+)�����c(H+)=c(OH-)��c(SO42-)������Һ��SO42-��ȫ������NaHSO4+Ba(OH)2= BaSO4��+H2O+NaOH����Ӧ������ΪNaOH���ʼ��ԣ�pH>7����3��������ԭBaCl2��Һ����СŨ��Ϊx,Ksp(BaSO4)= c(Ba2+)* c(SO42-),1.1��10-10=(0.02/2)*(x/2),���x=2.2��10-8mol/L��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������£�pH=5��NaHSO3��Һ�У�c(HSO3һ)��c(SO32һ)��c(H2SO3) |
| B����0.1mol��Lһ1NaHCO3��Һ���У� c(Na��) +c(H������c(HCO3һ) + c(CO32��) + c(H2CO3) |
| C����0.1mol��Lһ1Na2CO3��Һ��c((HCO3��) + 2c(H2CO3) +c(H��)��c(OH��) |
| D�������£���10mL0.lmol.Lһ1��������20mL0.1mol.Lһ1�İ�ˮ��ϣ�������Һ�У��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Һ�����ԣ���ѡ�ü��Ȼ��̪��ָʾ�� | B����Һ�����ԣ�ֻ��ѡ��ʯ����ָʾ�� |
| C����Һ�ʼ��ԣ���ѡ�ü��Ȼ��̪��ָʾ�� | D����Һ�ʼ��ԣ�ֻ��ѡ�÷�̪��ָʾ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���Ѵ�ˮ���ȣ�ˮ�ĵ���̶�����ˮ��Ȼ�����Եģ�pH=7 |
| B���ڴ�����Һ�м���������CH3COONa(s)�����pH�����Ҫԭ����CH3COONaˮ��ʼ��ԣ��кʹ�������H+ |
| C��pH=3�Ĵ�����Һ��ϡ����10����pH<4 |
| D����ͬŨ�ȵ�CH3COONa��Һ��Na2CO3��Һ��ȣ�Na2CO3��Һ��pHС |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
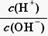 ��1010����ش��������⣺
��1010����ش��������⣺A��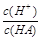 | B��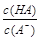 | C��c(H��)��c(OH��) | D��c(OH��) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��3.0��3.3 | B��3.3��3.5 | C��3.5��4.0 | D��3.7��4.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��c(SO42-)��c(H+)��c(NH4+)��c(OH-) | B��c(H+)��c(SO42-)��c(NH4+)��c(OH-) |
| C��c(OH-)��c(NH4+)��c(H+)��2c(SO42-) | D��c(OH-)+c(NH3��H2O)��c(H+)��c(SO42-) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| �ⶨ��� | ������Һ�����/mL | ���������Һ�����/mL | |
| �ζ�ǰ | �ζ��� | ||
| 1 | 20.00 | 0.50 | 20.78 |
| 2 | 20.00 | 1.20 | 21.32 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢ� | B���ڢۢ� | C���٢ۢ� | D���٢ڢۢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com