
����Ŀ��ʵ�������ܶ�Ϊ1.19 g/mL����������Ϊ36.5%��Ũ��������500 mL 0.4 mol/L���ᡣ�ش��������⣺
(1)��Ũ������HCl�����ʵ���Ũ��Ϊ______��
(2)��������Ũ���������ˮ����500 mL 0.4 mol/L���ᡣ
������ȡ______mL����Ũ����������ơ�
�ڸ�����ʵ��������Ҫ����Ҫ������������Ͳ���ձ�����������_____________��
��������Ũ��������0.4 mol/L���ᣬ���ݵIJ�����_____________________��
(3)����500 mL 0.4 mol/L����ʱ,���в����лᵼ�½��ƫ�͵���____________(�����)��
a.����Ͳ��ȡŨ����ʱ������Ͳ�Ŀ̶�
b.����Ͳ��ȡŨ�����ϴ����Ͳ������ϴ��Һת������ƿ��
c.ҡ�Ⱥ���Һ����ڿ̶��ߣ��ּ�ˮ���̶���
d.����������Һ������ƿת�Ƶ��Լ�ƿ��ʱ��������Һ�彦��
���𰸡�11.9 mol/L 16.8 ��ͷ�ιܺ�500 mL����ƿ �ز�����������ƿ��עˮ������̶���1��2 cm�������ý�ͷ�ιܣ��μ���Һ��İ�Һ����ʹ���̶������� c
��������
��1��Ũ��������ʵ���Ũ��Ϊ��![]() mol/L��
mol/L��
��2��������Ũ��������ΪV������V��11.9mol/L=500mL��0.4mol/L����V=16.8mL�����Ƹ���Һ��Ҫ�IJ�������Ϊ���ձ�����������500mL����ƿ����ͷ�ιܣ����ݵľ������Ϊ���ز�����������ƿ��עˮ������̶���1��2 cm�������ý�ͷ�ιܣ��μ���Һ��İ�Һ����ʹ���̶������С�
��3������a�У�����Ͳ��ȡŨ����ʱ������Ͳ�Ŀ̶ȣ���ȡ��Ũ����ƫ�࣬Ũ��ƫ����b�У�����Ͳ��ȡŨ�����ϴ����Ͳ������ϴ��Һת������ƿ�У����ʵ����ʵ������ӣ�Ũ��ƫ�ߣ�����c�У�ҡ�Ⱥ���Һ����ڿ̶��ߣ��ּ�ˮ���̶��ߣ�ˮ�Ӷ࣬��Ũ��ƫС������d�У�����������Һ������ƿת�Ƶ��Լ�ƿ��ʱ��������Һ�彦����Ũ�Ȳ�Ӱ�졣


 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(14��) ����25 ��ʱ0.1 mol/L�İ�ˮ����ش��������⣺
(1)����ˮ�м�����������粒��壬��ʱ��Һ�� c(OH��) / c(NH3��H2O) ______ (��������������С������������)��
(2)����ˮ�м���ϡ���ᣬʹ��ǡ���кͣ�д����Ӧ�����ӷ���ʽ��
_____________________________________________________________________��
������Һ��pH________7(����>����<����������)�������ӷ���ʽ��ʾ��ԭ��
_________________ ��
(3)����ˮ�м���ϡ��������Һ��pH��7����ʱc(NH4��)��a mol/L����c(SO42��)��________��
(4)����ˮ�м���pH��1�����ᣬ�Ұ�ˮ������������Ϊ1��1����������Һ�и��������ʵ���Ũ���ɴ�С�Ĺ�ϵ��______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��п�̵���������е������ܴ�����п�̵�أ��ɵ�أ��ͼ���п�̵�صĹ�����ͼ��ʾ�������й�˵��������ǣ� ��

A. ���߷ֱ�����6.5gп������ת��0.2mol����
B. ���ߵ�������Ӧʽ��Ϊ![]()
C. ����п�̵�ر�����п�̵�ر�������
D. ����п�̵������©Һ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��Ӧ2CH3OH(g)![]() CH3OCH3(g)��H2O(g)����ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£����ܱ������м���CH3OH����Ӧ���е�ijʱ�̲�ø���ֵ�Ũ�����£�
CH3OCH3(g)��H2O(g)����ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£����ܱ������м���CH3OH����Ӧ���е�ijʱ�̲�ø���ֵ�Ũ�����£�
���� | CH3OH | CH3OCH3 | H2O |
Ũ��/mol��L��1 | 0.44 | 0.6 | 0.6 |
������������ȷ����(����)
A. ����CH3OH��Ũ�ȣ���ʹ����Ӱٷ������࣬��Ӧ���ʼӿ�
B. ��ʱ�������淴Ӧ���ʵĴ�С��v����v��
C. ƽ��ʱc(CH3OH)��0.04 mol��L��1
D. ������CH3OH����10 min��Ӧ�ﵽƽ�⣬��ʱ���ڷ�Ӧ����v(CH3OH)��1.6 mol��L��1��min��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮFeCl3���غ�ɫ������FeCl3���׳��⣬100������ʱ������ijѧϰС��������ͼװ��(ijЩ�г�������ȥ)�Ʊ����ռ���ˮFeCl3���塣��ش�
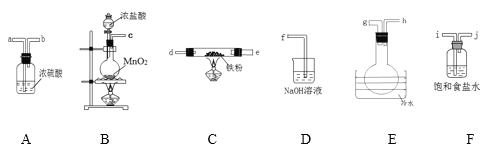
(1)�����������װ���������ӵĺ���˳��Ϊ__________________(�������ӿڵ���ĸ������װ�ÿ��ظ�ʹ��)��
(2)���Ӻø�װ�ý���ʵ�飬ʵ�鲽�����£����װ�������Ժ�װ��ҩƷ��________(�밴��ȷ��˳���������в�������)��
�ټ���Fe�۷�Ӧһ��ʱ���ڴ�Һ©������ͨһ��ʱ������
�۹رշ�Һ©������ ��ֹͣ���ȣ������ȴ
(3)װ��F������Ϊ_____________________��װ��E����ˮԡ������Ϊ__________��
(4)д��װ��B����������Ӧ�Ļ�ѧ����ʽ______________________________��
(5)д��װ��D����������Ӧ�����ӷ���ʽ______________________________��
(6)����װ��B�в���������ͨ��ˮ�У�������Ӧ�����ӷ���ʽΪ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ�������ʵ�鷽�����Է���KCl��BaCl2���ֹ�������Իش��������⣺
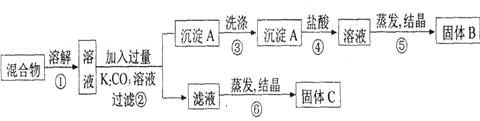
��1��B���ʵĻ�ѧʽΪ_____________________��
��2���÷���������ʧ������ijһ����Ʋ�����ʹ������ﲻ�����ò�����_______������ţ��Ľ���ʩ��__________________________��
��3��д��K2CO3�ĵ��뷽��ʽ_________________________________________________��
��4��д���ڢ������ж�Ӧ�����ӷ���ʽ��
��_______________________________________________________________
��_______________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з��ӻ�����������ԭ�Ӽ۲���ӶԼ��ι���Ϊ�������ҷ��ӻ����ӿռ�Ĺ���ΪV�ε��ǣ� ��
A. NH4+ B. PH3 C. H3O+ D. OF2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������Ӿ������幹��ʾ��ͼ����ͼ��ʾ��

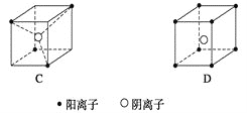
(1)��M���������ӣ���N���������ӣ�д�������Ӿ������ɱ���ʽ��
A��__________��B��________��C��________��D��__________________________��
(2)��֪FeS2����(���������Ҫ�ɷ�)����A������ṹ��
��FeS2�����о��еĻ�ѧ��������__________________________________________��
��������ṹA�����ڵ����������Ӽ�ľ���Ϊacm������NA���������ӵ���������FeS2������ܶ���________g��cm��3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и��������У���������ͼ��ʾת����ϵ���ǣ���Ӧ������ȥ����ͷ��ʾһ��ת������ ��
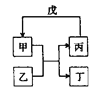
ѡ�� | �� | �� | �� | �� |
A | NH3 | Cl2 | N2 | H2 |
B | C | SiO2 | CO | CuO |
C | Al��OH��3 | NaOH | NaAlO2 | CO2 |
D | Br2 | FeI2 | FeBr3 | Cl2 |
A. AB. BC. CD. D
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com