


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꼪��ʡ�߿���ϰ������⣨���ۣ���ѧ���� ���ͣ�ѡ����
����������������Һ���й���������ȷ����
|
|
�� |
�� |
�� |
�� |
|
Ũ��c/mol/L |
0.1 |
0.1 |
0.1 |
0.1 |
|
��Һ |
��ˮ |
CH3COONa��Һ |
���� |
���� |
A����20mL����Һ����μ������Һ����Һ���������仯����ͼ��1����ʾ
B���ڡ�������Һ�������ϣ�����Ũ�ȣ�2c(Na+)=c(CH3COO-)+c(CH3COOH)
C���â۵ζ��٣��÷�̪��ָʾ�����ζ���������ͼ��2����ʾ��
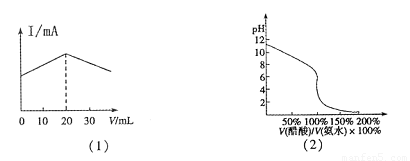
D���١�������Һ�������ϣ�����Ũ�ȣ�c(Cl-)>c(NH4+)>c(H+)>c(OH-)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ�������ѡ����

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com