

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| Ź±¼ä£Øs£© C£Ømol/L£© |
0 |
20 |
40 |
60 |
80 |
100 |
| C£ØN2O4£© | 0.20 | a | 0.10 | c | d | e |
| C£ØNO2£© | 0.00 | 0.12 | b | 0.22 | 0.22 | 0.22 |
| c2(NO2) |
| c(N2O4) |
| c2(NO2) |
| c(N2O4) |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| Ź±¼ä£Øs£© | 0 | 20 | 40 | 60 | 80 | 100 |
| n£ØN2O4£©£Ømol£© | 0.40 | a | 0.20 | c | d | e |
| n£ØNO2£©£Ømol£© | 0.00 | 0.24 | b | 0.52 | 0.60 | 0.60 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
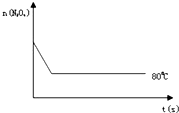 ŅŃÖŖN2O4£ØĪŽÉ«£©?2NO2 £Øŗģ×ŲÉ«£©£¬ŌŚ80”ꏱ£¬½«0.80molµÄN2O4ĘųĢå³äČė4LŅŃ¾³éæյĹĢ¶ØČŻ»żµÄĆܱÕČŻĘ÷ÖŠ£¬øōŅ»¶ĪŹ±¼ä¶ŌøĆČŻĘ÷ÄŚµÄĪļÖŹ½ųŠŠ·ÖĪö£¬µĆµ½ČēĻĀŹż¾Ż£ŗ
ŅŃÖŖN2O4£ØĪŽÉ«£©?2NO2 £Øŗģ×ŲÉ«£©£¬ŌŚ80”ꏱ£¬½«0.80molµÄN2O4ĘųĢå³äČė4LŅŃ¾³éæյĹĢ¶ØČŻ»żµÄĆܱÕČŻĘ÷ÖŠ£¬øōŅ»¶ĪŹ±¼ä¶ŌøĆČŻĘ÷ÄŚµÄĪļÖŹ½ųŠŠ·ÖĪö£¬µĆµ½ČēĻĀŹż¾Ż£ŗ| Ź±¼ä£ØS£© | 0 | 20 | 40 | 60 | 80 | 100 |
| n£Ø N2O4 £©£Ømol£© | 0.80 | a | 0.40 | c | d | e |
| n£ØNO2£©£Ømol£© | 0.00 | 0.48 | b | 1.04 | 1.20 | 1.20 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| Ź±¼ä/s n/mol |
0 | 20 | 40 | 60 | 80 | 100 |
| n£ØN2O4£© | 0.40 | a | 0.20 | c | d | e |
| n£ØNO2£© | 0.00 | 0.24 | b | 0.52 | 0.60 | 0.60 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| Ź±¼ä/s | 0 | 30 | 60 | 90 |
| n£ØL£©/mol | 0.80 | a | b | c |
| n£ØM£©/mol | 0.00 | 0.10 | 0.20 | 0.20 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com