
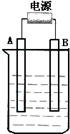
 ŌŚČēĶ¼ÓĆŹÆÄ«×÷µē¼«µÄµē½ā³ŲÖŠ£¬·ÅČė500mLŗ¬Ņ»ÖÖČÜÖŹµÄijĄ¶É«ČÜŅŗ½ųŠŠµē½ā£¬¹Ū²ģµ½Aµē¼«±ķĆęÓŠŗģÉ«µÄ¹ĢĢ¬ĪļÖŹÉś³É£¬Bµē¼«ÓŠĪŽÉ«ĘųĢåÉś³É£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
ŌŚČēĶ¼ÓĆŹÆÄ«×÷µē¼«µÄµē½ā³ŲÖŠ£¬·ÅČė500mLŗ¬Ņ»ÖÖČÜÖŹµÄijĄ¶É«ČÜŅŗ½ųŠŠµē½ā£¬¹Ū²ģµ½Aµē¼«±ķĆęÓŠŗģÉ«µÄ¹ĢĢ¬ĪļÖŹÉś³É£¬Bµē¼«ÓŠĪŽÉ«ĘųĢåÉś³É£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
| ||
| ||
| 1.6g |
| 64g/mol |
| ||
| 0.05mol |
| 0.5L |
| 22.4L |
| 22.4L/mol |
| 22.4L |
| 22.4L/mol |
| 1mol |
| 1L |


 »Ŗ¶«Ź¦“ó°ęŅ»æĪŅ»Į·ĻµĮŠ“š°ø
»Ŗ¶«Ź¦“ó°ęŅ»æĪŅ»Į·ĻµĮŠ“š°ø ĆĻ½ØĘ½ĆūŠ£æ¼¾ķĻµĮŠ“š°ø
ĆĻ½ØĘ½ĆūŠ£æ¼¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ŌŚČēĶ¼ÓĆŹÆÄ«×÷µē¼«µÄµē½ā³ŲÖŠ£¬·ÅČė500mLŗ¬Ņ»ÖÖČÜÖŹµÄijĄ¶É«ČÜŅŗ½ųŠŠµē½ā£¬¹Ū²ģµ½Aµē¼«±ķĆęÓŠŗģÉ«µÄ¹ĢĢ¬ĪļÖŹÉś³É£¬Bµē¼«ÓŠĪŽÉ«ĘųĢåÉś³É£»µ±ČÜŅŗÖŠµÄŌÓŠČÜÖŹĶźČ«µē½āŗó£¬Ķ£Ö¹µē½ā£¬Č”³öAµē¼«£¬Ļ“µÓ”¢øÉŌļ”¢³ĘĮ攢µē¼«ŌöÖŲ1.6g£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
ŌŚČēĶ¼ÓĆŹÆÄ«×÷µē¼«µÄµē½ā³ŲÖŠ£¬·ÅČė500mLŗ¬Ņ»ÖÖČÜÖŹµÄijĄ¶É«ČÜŅŗ½ųŠŠµē½ā£¬¹Ū²ģµ½Aµē¼«±ķĆęÓŠŗģÉ«µÄ¹ĢĢ¬ĪļÖŹÉś³É£¬Bµē¼«ÓŠĪŽÉ«ĘųĢåÉś³É£»µ±ČÜŅŗÖŠµÄŌÓŠČÜÖŹĶźČ«µē½āŗó£¬Ķ£Ö¹µē½ā£¬Č”³öAµē¼«£¬Ļ“µÓ”¢øÉŌļ”¢³ĘĮ攢µē¼«ŌöÖŲ1.6g£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ŌŚČēĶ¼ÓĆŹÆÄ«×÷µē¼«µÄµē½ā³ŲÖŠ£¬·ÅČė500mLŗ¬Ņ»ÖÖČÜÖŹµÄijĄ¶É«ĮņĖįŃĪČÜŅŗ½ųŠŠµē½ā£¬¹Ū²ģµ½Aµē¼«±ķĆęÓŠŗģÉ«µÄ¹ĢĢ¬ĪļÖŹÉś³É£¬Bµē¼«ÓŠĪŽÉ«ĘųĢåÉś³É£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
ŌŚČēĶ¼ÓĆŹÆÄ«×÷µē¼«µÄµē½ā³ŲÖŠ£¬·ÅČė500mLŗ¬Ņ»ÖÖČÜÖŹµÄijĄ¶É«ĮņĖįŃĪČÜŅŗ½ųŠŠµē½ā£¬¹Ū²ģµ½Aµē¼«±ķĆęÓŠŗģÉ«µÄ¹ĢĢ¬ĪļÖŹÉś³É£¬Bµē¼«ÓŠĪŽÉ«ĘųĢåÉś³É£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚČēĶ¼ÓĆŹÆÄ«×÷µē¼«µÄµē½ā³ŲÖŠ£¬·ÅČė500mLŗ¬Ņ»ÖÖČÜÖŹµÄijĄ¶É«ĮņĖįŃĪČÜŅŗ½ųŠŠµē½ā£¬¹Ū²ģµ½Aµē¼«±ķĆęÓŠŗģÉ«µÄ¹ĢĢ¬ĪļÖŹÉś³É£¬Bµē¼«ÓŠĪŽÉ«ĘųĢåÉś³É”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ĒėŠ“³öB¼«°åµÄĆū³Ę£ŗ µē¼«·“Ó¦Ź½
Š“³öµē½āŹ±·“Ó¦µÄ×ÜĄė×Ó·½³ĢŹ½
£Ø2£©Čōµ±ČÜŅŗÖŠµÄŌÓŠČÜÖŹĶźČ«µē½āŗó£¬Ķ£Ö¹µē½ā£¬Č”³öAµē¼«£¬Ļ“µÓ”¢øÉŌļ”¢³ĘĮ攢µē¼«ŌöÖŲ1.6g”£µē½āŗóČÜŅŗµÄpHĪŖ £»ŅŖŹ¹µē½āŗóČÜŅŗ»Öø“µ½µē½āĒ°µÄדĢ¬£¬ŌņŠč¼ÓČė £¬ĘäÖŹĮæĪŖ g”££Ø¼ŁÉčµē½āĒ°ŗóČÜŅŗµÄĢå»ż²»±ä£©
£Ø3£©ČōŌČÜŅŗĪŖ1L K2SO4”¢CuSO4µÄ»ģŗĻČÜŅŗ£¬ĒŅc£ØSO42-£©= 2.0mol/L £»ČēĶ¼×°ÖƵē½ā£¬µ±Į½¼«¶¼ŹÕ¼Æµ½22.4LĘųĢå£Ø±ź×¼×“æö£©Ź±£¬Ķ£Ö¹µē½ā”£
ŌņŌČÜŅŗÖŠµÄc(K+)£½
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģŗÓ±±Ź”øßŅ»ĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
ŌŚČēĶ¼ÓĆŹÆÄ«×÷µē¼«µÄµē½ā³ŲÖŠ£¬·ÅČė500 mLŗ¬Ņ»ÖÖČÜÖŹµÄijĄ¶É«ČÜŅŗ½ųŠŠµē½ā£¬¹Ū²ģµ½Aµē¼«±ķĆęÓŠŗģÉ«µÄ¹ĢĢ¬ĪļÖŹÉś³É£¬Bµē¼«ÓŠĪŽÉ«ĘųĢåÉś³É£»µ±ČÜŅŗÖŠµÄŌÓŠČÜÖŹĶźČ«µē½āŗó£¬Ķ£Ö¹µē½ā£¬Č”³öAµē¼«”¢Ļ“µÓ”¢øÉŌļ”¢³ĘĮ棬µē¼«ŌöÖŲ1.6 g”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ

(1)Bµē¼«·¢Éś·“Ó¦µÄµē¼«·“Ó¦Ź½_________________________”£
(2)Š“³öµē½āŹ±·“Ó¦µÄĄė×Ó·½³ĢŹ½_________________________________________”£
(3)µē½āŗóČÜŅŗµÄH+µÄĪļÖŹµÄĮæÅضČĪŖ________£¬ŅŖŹ¹µē½āŗóČÜŅŗ»Öø“µ½µē½āĒ°µÄדĢ¬£¬ŌņŠč¼ÓČė________£¬ĘäÖŹĮæĪŖ________g”£(¼ŁÉčµē½āĒ°ŗóČÜŅŗµÄĢå»ż²»±ä)
(4)ŌČÜŅŗÖŠæÉÄÜŗ¬ÓŠµÄĖįøłĄė×ÓĪŖ£Ø £©
A CO32- B Cl- C SO42-
Éč¼ĘŹµŃé¼ģŃéøĆĖįøłĄė×Ó£¬Š“³ö²Ł×÷²½Öč£¬ŹµŃéĻÖĻóŗĶŹµŃé½įĀŪ________________________
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com