
 NH4++OH-�����ж�NH3����ˮ���γ�NH3?H2O�ĺ����ṹ��______������ĸ����
NH4++OH-�����ж�NH3����ˮ���γ�NH3?H2O�ĺ����ṹ��______������ĸ����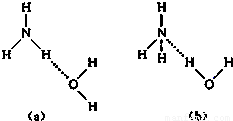



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������и��⣺
(1)ǰ������Ԫ���У���̬ԭ����δ�ɶԵ���������������������ͬ��Ԫ����________�֡�
(2)�ڢ�A����A��Ԫ����ɵĻ�����GaN��GaP��GaAs�����˹��ϳɵ����Ͱ뵼����ϣ��侧��ṹ�뵥�������ơ�Gaԭ�ӵĵ����Ų�ʽΪ______________����GaN�����У�ÿ��Gaԭ����__________��Nԭ����������ͬһ��Gaԭ��������Nԭ�ӹ��ɵĿռ乹��Ϊ________�����Ĵ��������У�GaN����_______���塣
(3)�ڼ��Է���NCl3�У�Nԭ�ӵĻ��ϼ�Ϊ-3,Clԭ�ӵĻ��ϼ�Ϊ+1,���Ʋ�NCl3ˮ�����Ҫ������_________(�ѧʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������и��⣺
(1)ǰ4�����У���̬ԭ����δ�ɶԵ�����Ϊ4��Ԫ��ԭ�ӵĵ����Ų�ʽΪ________________��
(2)NaCl�����У���Na+�Ⱦ����������Na+��________________����
(3)N ԭ�ӵĵ�һ������___________(�� ������������ ��=��)Oԭ�ӵĵ�һ�����ܣ�N2�����д���_______���Ҽ���_______���м���NH3�ķе��PH3_________(��ߡ��͡�)��![]() ��Nԭ�ӵ��ӻ���ʽΪ________________��
��Nԭ�ӵ��ӻ���ʽΪ________________��![]() �Ŀռ乹��Ϊ________________��
�Ŀռ乹��Ϊ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�긣��ʡ���ݰ�һ�С���Ϫһ�и߶���ѧ������������ѧ�Ծ� ���ͣ������
һ�������£������Ϊ3 L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����CO(g)��2H2(g) 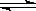 CH3OH(g)����������������и��⣺
CH3OH(g)����������������и��⣺

(1) �жϸ÷�Ӧ�ﵽƽ��״̬�ı�־�� ��������ĸ��
a��CO��CH3OHŨ�����
b��CO�ٷֺ������ֲ���
c�������������ѹǿ����
d��CH3OH������������CO�������������
e�������л��������ܶȱ��ֲ���
(2) �����CO��ת���ʣ����д�ʩ���е���
��������ĸ��
a����װ�����ٳ���N2 b����װ�����ٳ���H2
c���ı䷴Ӧ�Ĵ��� d�������¶�
(3) ��Ӧ�ﵽƽ��ʱ��ƽ�ⳣ������ʽK�� �������¶ȣ�Kֵ �����������С�����䡱����
(4) ��500�棬�ӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v(H2)��
(5) �������������������£��Դ���E�����ϵ���ѹ����ԭ����1/2�������йظ���ϵ��˵����ȷ����
a��������Ũ�ȼ��� b������Ӧ���ʼӿ죬�淴Ӧ����Ҳ�ӿ�
c���״������ʵ������� d������ƽ��ʱn(H2)/n(CH3OH)����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
(10��)һ�������£������Ϊ3 L���ܱ������У�һ����̼��������Ӧ���ɼ״�(����ΪCu2O/ZnO)��CO(g)��2H2(g) CH3OH(g)
CH3OH(g)

��������������и��⣺
(1)��Ӧ�ﵽƽ��ʱ��ƽ�ⳣ������ʽK�� �������¶ȣ�Kֵ (���������С�����䡱)��
(2)��500 �棬�ӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v (H2)�� ��
(3)�������������������£��Դ���E�����ϵ���ѹ����ԭ����1/2�������йظ���ϵ��˵����ȷ����
a��������Ũ�ȼ��� b������Ӧ���ʼӿ죬�淴Ӧ����Ҳ�ӿ�
c���״������ʵ������� d������ƽ��ʱn(H2)/n(CH3OH)����
(4)���о�����Ӧ������������õ�ΪCu2O����Ӧ��ϵ�к�����CO2������ά�ִ���Cu2O�������䣬ԭ���ǣ�______________________________ (�û�ѧ����ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012������ʡ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��15�֣�һ�������£������Ϊ3 L���ܱ������У�һ����̼��������Ӧ���ɼ״�(����ΪCu2O/ZnO)��CO(g)��2H2(g) CH3OH(g)
CH3OH(g)

��������������и��⣺
(1)��Ӧ�ﵽƽ��ʱ��ƽ�ⳣ������ʽK�� �������¶ȣ�Kֵ (���������С�����䡱)��
(2)��500 �棬�ӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v (H2)�� ��
(3)�������������������£��Դ���E�����ϵ���ѹ����ԭ����1/2�������йظ���ϵ��˵����ȷ����
a��������Ũ�ȼ��� b������Ӧ���ʼӿ죬�淴Ӧ����Ҳ�ӿ�
c���״������ʵ������� d������ƽ��ʱn(H2)/n(CH3OH)����
(4)���о�����Ӧ������������õ�ΪCu2O����Ӧ��ϵ�к�����CO2������ά�ִ���Cu2O�������䣬ԭ���ǣ�_____________________________(�û�ѧ����ʽ��ʾ)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com