
��������ĵ���ƽ�ⳣ�����±���
| ���� | HCOOH | HCN | H2CO3 |
| ����ƽ�ⳣ�� ��25�棩 | Ki=1��77��10-4 | Ki=4��9��10-10 | Ki1=4��3��10-7 Ki2=5��6��10-11 |
����ѡ��������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | HCOOH | HCN | H2CO3 |
| ����ƽ�ⳣ����25�棩 | Ki=1.77��10-4 | Ki=4.9��10-10 | Ki1=4.3��10-7 Ki2=5.6��10-11 |
| A��2CN-+H2O+CO2��2HCN+CO32- |
| B��2HCOOH+CO32-��2HCOO-+H2O+CO2�� |
| C���к͵��������pH��HCOOH��HCN����NaOH����ǰ��С�ں��� |
| D�����������Ũ�ȵ�HCOONa��NaCN��Һ��������������ǰ��С�ں��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�������ʡ�����и���10���¿���ѧ�Ծ����������� ���ͣ�ʵ����
��14�֣���֪�������ճ������г��������ᡣ
(1)��pH��ֽ�ⶨ����pH�IJ�����___________��
(2)�������� pH =5�Ĵ���ϡ��Һ�У�����������c(H+)�ľ�ȷֵ��______ mol?L��1��
(3)��0.1000 mol?L��1NaOH��Һ�ζ�20.00mLijŨ�ȵ�CH3COOH��Һ�����ֲ������£�
��ȡһ֧������ˮϴ���ļ�ʽ�ζ��ܣ����������������Һ����¼��ʼ����
������ʽ�ζ��ܷų�һ��������Һ������������ˮϴ������ƿ�У�����2�μ���
�۵ζ�ʱ���ߵμӱ���ͬʱע�ӵζ�����Һ��ı仯
��ѡ������ʵ������еĴ���֮�� (�����)������ʵ����һ�����ʵ���Ũ����Һ����ʵ�����õ�����ͬ����___________________��
(4)ij�εζ�ǰ�ζ���Һ����ͼ��ʾ������Ϊ________mL��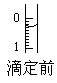
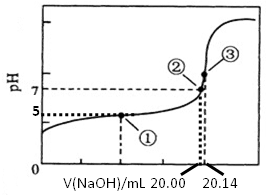
(5)������ȷʵ���������Ƶĵζ���������ͼ��ʾ�����е����ʾ��Һ��c(CH3COO-)=1.7c(CH3COOH)�������ʾ��Һ��c(CH3COO-)+c(CH3COOH)=c(Na+)���������ĵ���ƽ�ⳣ��___________��CH3COOH�����ʵ���Ũ��Ϊ__________ mol?L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�������ʡ����10���¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
��14�֣���֪�������ճ������г��������ᡣ
(1)��pH��ֽ�ⶨ����pH�IJ�����___________��
(2)�������� pH =5�Ĵ���ϡ��Һ�У� ����������c(H+)�ľ�ȷֵ��______ mol•L��1 ��
(3)��0.1000 mol•L��1NaOH��Һ�ζ�20.00mLijŨ�ȵ�CH3COOH��Һ�����ֲ������£�
��ȡһ֧������ˮϴ���ļ�ʽ�ζ��ܣ����������������Һ����¼��ʼ����
������ʽ�ζ��ܷų�һ��������Һ������������ˮϴ������ƿ�У�����2�μ���
�۵ζ�ʱ���ߵμӱ���ͬʱע�ӵζ�����Һ��ı仯
��ѡ������ʵ������еĴ���֮�� (�����)������ʵ����һ�����ʵ���Ũ����Һ����ʵ�����õ�����ͬ����___________________��
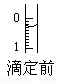
(4)ij�εζ�ǰ�ζ���Һ����ͼ��ʾ������Ϊ________mL��
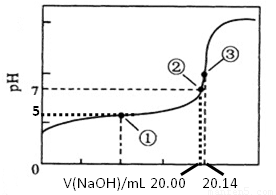
(5)������ȷʵ���������Ƶĵζ���������ͼ��ʾ�����е����ʾ��Һ��c(CH3COO-)=1.7c(CH3COOH)�������ʾ��Һ��c(CH3COO-)+c(CH3COOH)=c(Na+)���������ĵ���ƽ�ⳣ��___________��CH3COOH�����ʵ���Ũ��Ϊ__________ mol•L��1��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com