
(14·Ö)ĮņĖį¹¤ŅµÖŠ2SO2(g)£«O2(g)“߻ƼĮ”÷2SO3(g)£»¦¤H<0(·ÅČČ·“Ó¦)ÓŠ¹ŲŹµŃ鏿¾ŻČēĻĀ£ŗ
  Ń¹Ēæ Ń¹ĒæSO2µÄ ×Ŗ»ÆĀŹ ĪĀ¶Č | 1”Į105 Pa | 5”Į105 Pa | 10”Į105 Pa | 50”Į105 Pa | 100”Į105 Pa |
| 450 ”ę | 97.5% | 98.9% | 99.2% | 99.6% | 99.7% |
| 550 ”ę | 85.6% | 92.9% | 94.9% | 97.7% | 98.3% |
 µÄæÕĘųŹĒĪŖĮĖ________”£
µÄæÕĘųŹĒĪŖĮĖ________”££Ø14·Ö£©””(1)Ź¹Ę½ŗāÕżĻņŅĘ¶Æ£¬ĢįøßSO2µÄ×Ŗ»ÆĀŹ
(2)øßĪĀŹ¹·“Ó¦ĖŁĀŹ¼Óæģ£¬Ėõ¶ĢĮĖ“ļµ½Ę½ŗāĖłŠčµÄŹ±¼ä£¬µ«ŹĒ¶ŌSO2µÄ×Ŗ»Æ²»Ąū”””£
“߻ƼĮŌŚøĆĪĀ¶ČĻĀ»īŠŌ×īĒ棬“߻Ɗ§¹ū×ī¼Ń”£
(3)¼Óæģ·“Ó¦ĖŁĀŹ£¬ĒŅŹ¹Ę½ŗāÕżĻņŅĘ¶Æ£¬ÓŠĄūÓŚSO2×Ŗ»ÆŗĶSO3µÄÉś³É”£””
³£Ń¹Ź±SO2µÄ×Ŗ»ÆĀŹŅŃ¾ŗÜøߣ¬ĪŽ²ÉÓĆøßŃ¹µÄ±ŲŅŖ£¬æöĒŅ£¬²ÉÓĆøßŃ¹»¹ŹÜ¶ÆĮ¦”¢Éč±ø µČĢõ¼žµÄĻŽÖĘ£¬ĢįøßĮĖ³É±¾”£
µČĢõ¼žµÄĻŽÖĘ£¬ĢįøßĮĖ³É±¾”£
(4)ÓĆĖ®ĪüŹÕSO3Ņ׊Ī³ÉĖįĪķ£¬ĪüŹÕŠ§¹ū²ī£¬¶ųÓĆÅØH2SO4ĪüŹÕŌņ²»Ņ׊Ī³ÉĖįĪķ£¬ĪüŹÕŠ§¹ūŗĆ”£””
·ĄÖ¹æÕĘųĪŪČ¾”£
½āĪö



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(14·Ö)ĮņĖį¹¤ŅµÖŠ2SO2(g)£«O2(g)“߻ƼĮ”÷2SO3(g)£»¦¤H<0(·ÅČČ·“Ó¦)ÓŠ¹ŲŹµŃ鏿¾ŻČēĻĀ£ŗ
|
SO2µÄ ×Ŗ»ÆĀŹ ĪĀ¶Č | 1”Į105 Pa | 5”Į105 Pa | 10”Į105 Pa | 50”Į105 Pa | 100”Į105 Pa |
| 450 ”ę | 97.5% | 98.9% | 99.2% | 99.6% | 99.7% |
| 550 ”ę | 85.6% | 92.9% | 94.9% | 97.7% | 98.3% |
(1)ŌŚÉś²śÖŠ³£ÓĆ¹żĮæµÄæÕĘųŹĒĪŖĮĖ________”£
(2)øßĪĀ¶ŌøĆ·“Ó¦ÓŠŗĪÓ°Ļģ£æ________£¬Źµ¼ŹÉś²śÖŠ²ÉÓĆ400”«500 ”ęµÄĪĀ¶Č³żĮĖæ¼ĀĒĖŁĀŹŅņĖŲĶā£¬»¹æ¼ĀĒµ½________”£
(3)Ōö“óŃ¹Ēæ¶ŌÉĻŹö·“Ó¦ÓŠŗĪÓ°Ļģ£æ____£¬µ«¹¤ŅµÉĻÓÖ³£²ÉÓĆ³£Ń¹½ųŠŠ·“Ó¦£¬ĘäŌŅņŹĒ______________”£
(4)³£ÓĆÅØH2SO4¶ų²»ÓĆĖ®ĪüŹÕSO3ŹĒÓÉÓŚ___ ___£¬Ī²ĘųÖŠSO2±ŲŠė»ŲŹÕ£¬Ö÷ŅŖŹĒĪŖĮĖ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģɽ¶«Ź”Ī¢É½Ņ»ÖŠøßČż10ŌĀŌĀæ¼»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
(14·Ö)ĮņĖį¹¤ŅµĪ²ĘųÖŠ¶žŃõ»ÆĮņµÄŗ¬Į泬¹ż0.05%(Ģå»ż·ÖŹż)Ź±Šč¾“¦Ąķŗó²ÅÄÜÅÅ·Å”£Ä³Š£»ÆѧŠĖȤŠ”×éÓū²ā¶ØijĮņĖį¹¤³§ÅÅ·ÅĪ²ĘųÖŠ¶žŃõ»ÆĮņµÄŗ¬Į棬·Ö±š²ÉÓĆŅŌĻĀ·½°ø£ŗ
[¼×·½°ø]£ŗČēĶ¼ĖłŹ¾£¬Ķ¼ÖŠĘųĢåĮ÷Įæ¼ĘBÓĆÓŚ×¼Č·²āĮæĶعżµÄĪ²ĘųĢå»ż”£½«Ī²ĘųĶØČėŅ»¶ØĢå»żŅŃÖŖÅØ¶ČµÄµāĖ®ÖŠ²ā¶ØSO2µÄŗ¬Į攣µ±Ļ“ĘųĘæCÖŠČÜŅŗĄ¶É«ĻūŹ§Ź±£¬Į¢¼“¹Ų±Õ»īČūA”£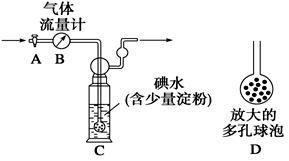
(1)Ļ“ĘųĘæCÖŠµ¼¹ÜÄ©¶ĖĮ¬½ÓŅ»øö¶ąæ×ĒņÅŻD£¬æÉŅŌĢįøߏµŃéµÄ×¼Č·¶Č£¬ĘäĄķÓÉŹĒ_______________________________________”£
(2)Ļ“ĘųĘæCÖŠČÜŅŗĄ¶É«ĻūŹ§ŗó£¬Ć»ÓŠ¼°Ź±¹Ų±Õ»īČūA£¬²āµĆµÄSO2ŗ¬Įæ____________(Ģī”°Ę«øß”±”¢”°Ę«µĶ”±»ņ”°ĪŽÓ°Ļģ”±)”£
[ŅŅ·½°ø]£ŗŹµŃé²½ÖčČēĻĀĆęĮ÷³ĢĶ¼ĖłŹ¾£ŗ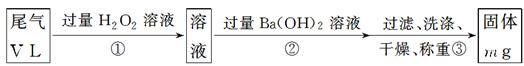
(3)²½Öč¢ŁÖŠ¹żĮæH2O2µÄ×÷ÓĆŹĒ
(4)Š“³ö²½Öč¢ŚÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½_______________________________________
(5)²½Öč¢ŚÖŠBa(OH)2ŹĒ·ń×ćĮæµÄÅŠ¶Ļ·½·ØŹĒ________________________________
(6)ĶعżµÄĪ²ĘųĢå»żĪŖV L(ŅŃ»»Ėć³É±ź×¼×“æö)Ź±£¬øĆĪ²ĘųÖŠ¶žŃõ»ÆĮņµÄŗ¬Įæ(Ģå»ż·ÖŹż)ĪŖ__________________________(ÓĆŗ¬ÓŠV”¢mµÄ“śŹżŹ½±ķŹ¾)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğŗžÄĻŹ”øßČżµŚČż“Ī½ĢÓżÖŹĮæ¼ģ²ā»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
(14·Ö)ŃĪĖį”¢ĮņĖįŗĶĻõĖį¶¼ŹĒÖŲŅŖµÄ»Æ¹¤ŌĮĻ£¬Ņ²ŹĒ»ÆѧŹµŃéŹŅĄļ±Ų±øµÄÖŲŅŖŹŌ¼Į”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©³£ĪĀĻĀ£¬æÉÓĆĢś”¢ĀĮÖʵÄČŻĘ÷Ź¢·ÅÅØĮņĖį£¬ĖµĆ÷ÅØĮņĖį¾ßÓŠ ŠŌ”£ÓĆ²£Į§°ōÕŗČ”ÅØĮņĖįµĪŌŚÖ½ÉĻ£¬Ö½Öš½„±äŗŚ£¬ĖµĆ÷ÅØĮņĖį¾ßÓŠ ŠŌ”£
£Ø2£©ĻõĖįĶŹĒÖʱøCu-Zn-AlĻµ“߻ƼĮµÄÖŲŅŖŌĮĻ£¬¹¤ŅµÉĻÓĆĻ“¾»µÄ·ĻĶŠ¼×÷ŌĮĻĄ“ÖʱøĻõĖįĶ”£ĻĀĮŠÖʱø·½·Ø·ūŗĻ”°ĀĢÉ«»Æѧ”±Ė¼ĻėµÄŹĒ £ØĢīŠņŗÅ£©”£
¢Ł Cu + HNO3£ØÅØ£©”ś Cu(NO3)2 ¢Ś Cu + HNO3£ØĻ”£©”ś Cu(NO3)2
¢Ū Cu CuO
CuO Cu(NO3)2
Cu(NO3)2
£Ø3£©¢ŁŌŚ100mL 18mol”¤L-1µÄÅØĮņĖįÖŠ¼ÓČė¹żĮæµÄĶʬ£¬¼ÓČČŹ¹Ö®³ä·Ö·“Ó¦£¬²āµĆ²śÉśµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»żæÉÄÜŹĒ ”£
A£®40.32L B£®30.24L C£®20.16L D£®13.44L
¢ŚČōŹ¹ÉĻŹö·“Ó¦¢ŁÖŠŹ£ÓąµÄĶʬ¼ĢŠųČܽā£¬æÉĻņĘäÖŠ¼ÓČėĻõĖįÄĘ£¬Š“³ö·“Ó¦µÄĄė×Ó·½³ĢŹ½ ”£
£Ø4£©Čō½«12.8gĶøśŅ»¶ØÖŹĮæµÄÅØHNO3·“Ó¦,ĶĻūŗÄĶźŹ±,¹²²śÉśĘųĢå5.6L(±ź×¼×“æö),ŌņĖłŗÄHNO3µÄĪļÖŹµÄĮæ mol
(5)ijĶ¬Ń§Ļņ½žÅŻĶʬµÄĻ”ŃĪĖįÖŠ¼ÓČėH2O2ŗó£¬ĶʬČܽā£¬²¢ĒŅøĆ·“Ó¦µÄ²śĪļÖ»ÓŠĀČ»ÆĶŗĶĖ®”£øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģÖŲĒģŹŠø߶žÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
(14·Ö)ĮņĖį¹¤ŅµÖŠ2SO2(g)£«O2(g)“߻ƼĮ”÷2SO3(g)£»¦¤H<0(·ÅČČ·“Ó¦)ÓŠ¹ŲŹµŃ鏿¾ŻČēĻĀ£ŗ
|
SO2µÄ ×Ŗ»ÆĀŹ ĪĀ¶Č |
1”Į105 Pa |
5”Į105 Pa |
10”Į105 Pa |
50”Į105 Pa |
100”Į105 Pa |
|
450 ”ę |
97.5% |
98.9% |
99.2% |
99.6% |
99.7% |
|
550 ”ę |
85.6% |
92.9% |
94.9% |
97.7% |
98.3% |
(1)ŌŚÉś²śÖŠ³£ÓĆ¹żĮæµÄæÕĘųŹĒĪŖĮĖ________”£
(2)øßĪĀ¶ŌøĆ·“Ó¦ÓŠŗĪÓ°Ļģ£æ________£¬Źµ¼ŹÉś²śÖŠ²ÉÓĆ400”«500 ”ęµÄĪĀ¶Č³żĮĖæ¼ĀĒĖŁĀŹŅņĖŲĶā£¬»¹æ¼ĀĒµ½________”£
(3)Ōö“óŃ¹Ēæ¶ŌÉĻŹö·“Ó¦ÓŠŗĪÓ°Ļģ£æ____£¬µ«¹¤ŅµÉĻÓÖ³£²ÉÓĆ³£Ń¹½ųŠŠ·“Ó¦£¬ĘäŌŅņŹĒ______________”£
(4)³£ÓĆÅØH2SO4¶ų²»ÓĆĖ®ĪüŹÕSO3ŹĒÓÉÓŚ___ ___£¬Ī²ĘųÖŠSO2±ŲŠė»ŲŹÕ£¬Ö÷ŅŖŹĒĪŖĮĖ________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com