
������Щ��ʵ��ʵ����˵�������ԣ�Cl2>Br2>I2__________________________________
(�����)��
����ˮ�ֱ����KBr��NaI��Һ����ɫ�������ˮ����NaCl��Һ�������Ա仯������KI������Һ�У���Һ����
��H2��Cl2�Ļ��������ձ�ը��H2��Br2�Ļ��������Ȳ��ܷ�Ӧ����H2��I2��Ӧ������
��Fe�ֱ���Cl2��Br2��I2��Ӧ����Fe�Ļ�����Ļ��ϼ۸ߵ�
��HCl��HBr��HI�����ȶ���Խ��Խ��
��Cl2��Br2��I2��ˮ�е��ܽ����С
 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ����(����)
A����������������������������ʹ�õĽ�������
B��������ͭ��Ӧ����㷺�Ľ�������
C���ѱ���Ϊ��21���͵Ľ�������Ӧ��ǰ���ܹ���
D��ͭ�ǵ����ԡ���������õ���ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��һ���������ǽ������ϣ�������ĥ����ʴ�������ȳ���ԡ��й������������£�

Ϊ��ȷ��C����ɣ�ijͬѧ���������µ�̽�����̡���֪F��G����������ˮ��ϡ����İ�ɫ������I�������ά��
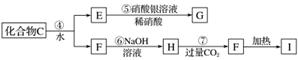
��Ҫ��ش��������⣺
(1)C�Ļ�ѧʽΪ________��X�Ļ�ѧʽΪ________��
(2)д�����з���ʽ��
��Ӧ�ڵĻ�ѧ����ʽ__________________________________________________��
��Ӧ�ߵ����ӷ���ʽ___________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��AԪ�ص�һ�ֵ�����һ����Ҫ�İ뵼����ϣ���AԪ�ص�һ�ֻ�����C��������������ܵ��ִ�ͨѶ����——���ά��C���ռӦ���ɺ�AԪ�صĻ�����D��
(1)��Ԫ�����ڱ��У�Aλ��________�壬��Aͬ�嵫���ԭ��������AС��Ԫ��B��ԭ�ӽṹʾ��ͼΪ______��A��B��ԭ�ӵĵ��Ӳ�ṹ�ϵ���ͬ����________________________
________________________________________________________________________��
(2)����C������ѧ��Ӧ������________(д����)����Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
(3)��C�봿���ϸ�������ʱҲ������ѧ��Ӧ����D��ͬʱ������B�����������E����ȫ����E��ȫ����D��������ˮ�л�Ϻ��ַ�����ѧ��Ӧ���ɺ�A�Ļ�����F��
��д������D��F�Ļ�ѧ��Ӧ����ʽ��____________________________________
________________________________________________________________________��
��Ҫ����������ۻ������������п�ѡ�õ���________��
A����ͨ�������� B��ʯӢ��������
C������������ D��������
(4)100 g C��ʯ��ʯ�Ļ�����ַ�Ӧ�����ɵ������ڱ�״���µ����Ϊ11.2 L,100 g�������ʯ��ʯ������������____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ˮ��ʯ��ʯ�ķ�Ӧ����ȡ��Ũ��HClO��Һ�ķ���֮һ��ijͬѧ������һ������������ȡHClO��Һ�����������¶���ʵ�飺
�����Թ��м�������Ŀ�״̼��ƣ��ټ���Լ20 mL������ˮ����ַ�Ӧ�����������ݲ�������Һ�Ļ���ɫ��ȥ��
�ڹ��ˣ�����Һ������ɫ�����ϣ�������Ư���Ը�ǿ��
��Ϊ��ȷ����Ӧ�������Һ��Ϊ���ݣ��ֱ��������ʵ�飺
��һ����ʯ��ˮ��ϣ���������������ɫ������
�ڶ�����ϡ�����ϣ����̲����������ݣ�
�����ݼ��ȣ�������Һ��������д�����ɫ���������
����⣬����ʵ���в�������ɫ�����ΪCO2���塣
(1)�Խ��Ϳ����ڱ�����ˮ�м���ʯ��ʯ�Ʊ�HClO��ԭ��________________________________________________________________________
________________________________________________________________________��
(2)д��������е�һ�ݼ��ڶ�����Һ������Ӧ�����ӷ���ʽ��
��һ��________________________________________________________________________��
�ڶ���________________________________________________________________________��
(3)�Ը�����ѧ֪ʶ�Ʋ⣬�ڢڵ���Һ�к��е����ʣ������ܽ�ļ����������⣬�����е���������Ϊ(д��ѧʽ)__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��SO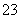 ��Fe2����Br����I����0.1 mol����Һ��ͨ���״���µ�Cl2��ͨ��Cl2���������Һ��������ӵ����ʵ����Ĺ�ϵͼ��ȷ����(����)
��Fe2����Br����I����0.1 mol����Һ��ͨ���״���µ�Cl2��ͨ��Cl2���������Һ��������ӵ����ʵ����Ĺ�ϵͼ��ȷ����(����)
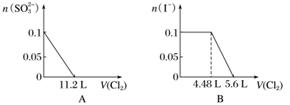
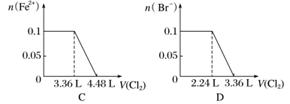
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ҫ��Br����ʽ�����ں�ˮ�У���ˮ�������ԡ���ҵ���Ʊ�Br2�IJ�������Ϊ
��һ�������£���Cl2ͨ��Ũ���ĺ�ˮ�У�����Br2
�������ȿ�����Br2����������ŨNa2CO3��Һ���գ�����NaBr��NaBrO3��
���������ữ����ڵõ��Ļ����
���������գ�
(1)Cl2����Br��Ӧ��__________�����½��У�Ŀ����Ϊ�˱���_________________��
(2)Br2�����ȿ�����������ԭ����________________________________________��
(3)д��������������Ļ�ѧ��Ӧ����ʽ___________________________________��
����������������ữ��ԭ�������______________������ڵIJ�Ʒ��ʱ���䵽Ŀ�ĵغ����ữ����Ҫ����Ϊ________________________________________________________
________________________________________________________________________��
(4)Ϊ�˳�ȥ��ҵBr2������Cl2������ҵBr2��
________________________________________________________________________
a��ͨ��HBr
b������Na2CO3��Һ
c������NaBr��Һ
d������Na2SO3��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ�У����������ӵķ�Ӧ��(����)
A��CO(g)��2H2(g)===CH3OH(g)
B��CaCO3��2HCl===CaCl2��H2O��CO2��
C��C(s)��O2(g)===CO2(g)
D��2Hg(l)��O2(g)===2HgO(s)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������Ľ���֮һ���������γɶ��������������������ࡣ���������̼��ˮ��ijһ�ܱ���ϵ�з�Ӧ������±���ʾ��
| ��ѧ��Ӧ | ƽ�ⳣ�� | �¶� | |
| 973K | 1 173K | ||
| ��Fe(s)��CO2(g)FeO(s)��CO(g) | K1 | 1.47 | 2.15 |
| ��Fe(s)��H2O(g)FeO(s)��H2(g) | K2 | 2.38 | 1.67 |
| ��CO(g)��H2O(g)CO2(g)��H2(g) | K3 | �� | �� |
���������գ�
(1)��Ӧ��Ϊ______(����ȡ����ȡ�)��Ӧ�����ݷ�Ӧ����ڿ����Ƶ���ͬ����K1��K2��K3֮��Ĺ�ϵ����K3��______(��K1��K2��ʾ)��
(2)973 Kʱ������Ӧ����һ���ݻ�Ϊ2 L�ķ�Ӧ������2 minʱ�ﵽƽ�⣬��3 mol���ӷ���ת�ƣ�����2 min��v(CO2)��____________����ѹ���������ݻ�Ϊԭ����һ�룬ƽ�⽫______�ƶ�(����������ҡ�����)��CO2��Ũ�Ƚ�______(���������С�����䡱)��ʹ�÷�Ӧ��ƽ��ת���ʼ�ƽ�ⳣ��������Ĵ�ʩ��________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com