
| A�� | ��ij��ɫ��Һ�еμ��Ȼ�����Һ��������ɫ�������ټ���ϡ�����ó������ܽ⣬˵��ԭ��Һ��һ������SO42- | |
| B�� | ij��Һ��ʹ���۵⻯����Һ�����������Һһ��Ϊ��ˮ������ˮ | |
| C�� | ��ij��ɫ�����ĩ�еμ�ϡ���ᣬ������ʹ����ʯ��ˮ����ǵ����壬��ԭ�����ĩ��һ����CO32-��HCO3- | |
| D�� | ��ij��ɫ�����ĩ����������Ũ��Һ���ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤��ԭ������һ������NH4+ |
���� A�����Ȼ�����Һ��Ӧ���ɵİ�ɫ��������Ϊ�Ȼ�����ԭ��Һ�в�һ��������������ӣ�
B����Ҫ������ǿ�ڵⵥ�ʣ����ܹ�ʱ���۵⻯����Һ����������Һ����Ϊ�Ȼ�������һ��Ϊ��ˮ����ˮ��
C����������Ҳ�ܹ�ʹ����ʯ��ˮ����ǣ�ԭ������ܺ�������������ӡ�������������ӣ���һ������CO32-����HCO3-���ӣ�
D��ʹʪ��ĺ�ɫʯ����ֽ����������Ϊ������ԭ��Һ��һ������笠����ӣ�
��� �⣺A����ij��ɫ��Һ�еμ��Ȼ�����Һ��������ɫ�������ð�ɫ��������Ϊ�Ȼ������ټ���ϡ������Ȼ����������ܽ⣬ԭ��Һ�п��ܴ��������ӣ���һ������SO42-���ӣ���A����
B��ij��Һ��ʹ���۵⻯����Һ����������Һ�к���������ǿ�ڵⵥ�ʵ����ʣ�����Ϊ�����ӣ���һ��Ϊ��ˮ������ˮ����B����
C����ij��ɫ�����ĩ�еμ�ϡ���ᣬ����ʹ����ʯ��ˮ����ǵ����壬���������Ϊ������������̼�����ܺ�������������ӣ�ԭ�����ĩ�в�һ������CO32-����HCO3-���ӣ���C����
D����ij��ɫ�����ĩ����������Ũ��Һ���ȣ�����ʹʪ��ĺ�ɫʯ����ֽ���������壬������һ��Ϊ��������֤��ԭ������һ������NH4+���ӣ���D��ȷ��
��ѡD��
���� ���⿼���˳������ӵļ��鷽������Ŀ�Ѷ��еȣ�ע�����ճ������Ӿ��еĻ�ѧ���ʣ���ȷ�������ӵļ��鷽�������������Ƿ����ʱ�������ų��������ӣ�������鷽���������ԣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 100mL0.1mol•L-1FeCl3��Һ�к���Fe3+����ĿΪ0.01NA | |
| B�� | ��ͭ�뵥����ķ�Ӧ�У�1molͭʧȥ�ĵ�����Ϊ2NA | |
| C�� | 15.6g��Na2S��Na2O2��ɵĹ��������У����е���������Ϊ0.2NA | |
| D�� | 104g����ϩ�� ���к���̼�������Ŀ��̼̼˫������Ŀ�ֱ�Ϊ8NA��4NA ���к���̼�������Ŀ��̼̼˫������Ŀ�ֱ�Ϊ8NA��4NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ҵ���Һ | B�� | CH3COONa��Һ | C�� | CH3COOH��Һ | D�� | ̼�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ֻ�Т٢� | B�� | ֻ�Тڢ� | C�� | �٢ڢۢ� | D�� | ֻ�Тۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H+ Na+ S2- CO32- | B�� | H+ Ca2+ MnO${\;}_{4}^{-}$ NO${\;}_{3}^{-}$ | ||
| C�� | K+ Ca2+ Cl- HCO${\;}_{3}^{-}$ | D�� | Cl- Mg2+ Al3+ OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ܢݢ� | B�� | �ڢۢܢ� | C�� | �٢ۢܢ� | D�� | �٢ڢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
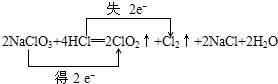
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������У�H20�ĵ���������� | B�� | c��Na+����c��HS-����c��H+����c��S2-����c��0H-�� | ||
| C�� | c��Na+��+c��H+���Tc��0H-��+c��HS-��+2c��S2-�� | D�� | c��Na+���Tc��HS-��+c��H2S�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com