
������KSCN(SCN���ǡ���±���ӡ�)��Һ���뵽�����ữ����������Һ�У���Һ��ɺ�ɫ�����ú�ɫ��Һ��Ϊ���ݣ���һ���м�������KMnO4��Һ����ɫ��ȥ��������һ����ͨ��SO2����ɫҲ��ȥ�������Ʋ�϶�����ȷ���� (����)��
A�����к�ɫ��ȥ��ԭ����KMnO4��SCN������
B�����к�ɫ��ȥ��ԭ����SO2��Fe3����ԭ��Fe2��
C�����к�ɫ��ȥ��ԭ����SO2��SCN����ԭ
D��SCN�����ʵ������¿�ʧȥ���ӱ�����Ϊ(SCN)2

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ʵ�к�ˮ��������Ӧ��������ˮ�����з�������ԭ������ȫ�����е��� (����)��
A��������ʹ��ˮ�е��γ�������������
B������̫����ʹ��ˮ���£�ͨ�������ʹ�䵭��
C������ˮ�������������Ի�ȡ��ˮ
D������ˮͨ�����ӽ�����֬�Գ�ȥ���������Ӽ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijϡ��Һ�к���Fe(NO3)3��Cu(NO3)2��HNO3�����������������ۣ���Һ��Fe2��Ũ�Ⱥͼ������۵����ʵ���֮��Ĺ�ϵ����ͼ��ʾ����ϡ��Һ��Fe(NO3)3��Cu(NO3)2��HNO3���ʵ���Ũ��֮��Ϊ (����)��

A��1��1��1�� B��1��3��1
C��3��3��8�� D��1��1��4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�⻯��ͭ(CuH)��һ�ֲ��ȶ������ʣ�����������ȼ�գ�Ҳ�����ᷴӦ����CuSO4��Һ�͡�ij���ʡ���40��50 ��ʱ��Ӧ������CuH�����������д������ (����)��
A����ij���ʡ����л�ԭ��
B��CuH�����ᷴӦ���ܲ���H2
C��CuH������ϡ���ᷴӦ��CuH��3H����NO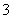 ===Cu2����NO����2H2O
===Cu2����NO����2H2O
D��CuH��������ȼ�գ�CuH��Cl2===CuCl��HCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й�������������ȷ���� (����)��
�����ܱ������������������ױ���ʴ�����������ڵ�Ѫ�쵰���к�����Ԫ�ء�����λ��Ԫ�����ڱ��е������ڵڢ�B�塡�������������о���ȼ�գ���������ˮ������ȼ�ա�������ǿ���������ᷴӦ�IJ������Fe(NO3)3������ͨ�����Ϸ�Ӧ�Ƶ�FeCl2��Fe(OH)3
A���٢ۡ� B���ڢܡ�
C���ڢݡ� D���ܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Fe3O4����Ҫ�Ļ�ѧ�Լ���������������ȼ������ȡ����Ϊ�����Ϳ�ݵķ�����ͼ1����ȡ������������ϵ��װ�ã�Aװ��������ȡ������̼���壬��Ҫ�������ȶ������ٿɿء�
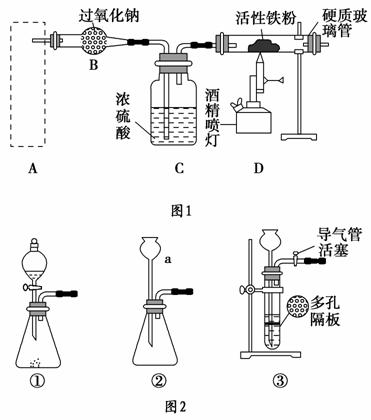
��ش��������⣺
(1)ͼ2������a��������________��
(2)������ĿҪ����ͼ2�����ѡ��________(�����)��ΪAװ�á�
(3)��ͼ2��װ�âٽ��������Լ��ķ�����________�����Һ©���м�����ˮ����һ�ᣬˮ�����µΣ�˵��װ�âٵ����������á�
(4)��Bװ���з�������Ҫ��Ӧ�Ļ�ѧ����ʽ��_________________________ _______________________________________________________________________________________________________________________��
(5)������������������Ԥ�ȵ����۽Ӵ�ʱ����Ӳ�ʲ������н��۲쵽������������________________________________________________________��
(6)��Ӧһ��ʱ�������Ӳ�ʲ������еĹ����ĩ�������ܽ⣬ȡ������Һ���Թ��У�������۵⻯����Һ��û����ɫ���֣��Ʋ�����ĩ�г�Fe3O4�⣬һ����_______________________________________________________��
(7)�����۱���ȫ���ģ�ijͬѧΪȷ�����������У�2������ȡ�������������Թ��У�����������ϡ�����ܽ⡣
��д���ܽ���̷�����Ӧ�����ӷ���ʽ______________________ ________________________________________________________��
��д��ȷ����2���������Լ����ơ��������衢ʵ������ͽ���_______________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ������NaOH��Һ����μ���AlCl3��Һ�����ɳ���Al(OH)3������AlCl3�������ı仯��ϵ��ͼ��ʾ���������������ڶ�Ӧ����Һ��һ���ܴ���������� (����)��
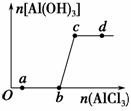
A��a���Ӧ����Һ�У�Na����Fe3����SO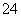 ��HCO
��HCO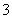
B��b���Ӧ����Һ�У�Na����S2����SO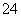 ��Cl��
��Cl��
C��c���Ӧ����Һ�У�Ag����Ca2����NO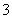 ��F��
��F��
D��d���Ӧ����Һ�У�K����NH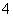 ��I����CO
��I����CO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ��ѧ�о���ѧϰС���ijNa2CO3��NaHCO3�����Һ����ɽ���̽����ȡ20.0 mL�û����Һ���ϼ���1.00 mol��L��1��ϡ���ᣬ�������������Ͳ�����������±���
| ��Ӧ�� | �� | �� | �� |
| �������x/mL | 0<x��10.0 | 10.0<x��40.0 | x>40.0 |
| ���� | ������ | �������� | ������ |
������Һ��c(HCO )Ϊ (����)��
)Ϊ (����)��
A��1.00 mol��L��1�� B��0.50 mol��L��1
C��1.50 mol��L��1�� D��2.00 mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ס��ҡ��������ֲ�����ͬ���ӵĿ����Ե���ʡ����������������±���ʾ��

ȡ�����������ֻ�����������ͬ�������Һ�����������ʵ���Ũ�ȣ�c���ף�>c���ң�>c���������������ʿ�����
��MgSO4 ��NaOH �ۣ�NH4��2SO4 ��Mg��NO3��2 ��NH4NO3
A���٢� B���ۢ� C���٢� D���ۢ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com