
| t/min | CO/mol | H2/mol | Z/mol |
| 1.00 | 1.00 | 0.00 | |
| 1 | 0.90 | 0.80 | 0.10 |
| 3 | 0.75 | 0.50 | 0.25 |
| 5 | 0.65 | 0.30 | 0.35 |
| 7 | 0.55 | 0.10 | 0.45 |
| 9 | 0.55 | 0.10 | 0.45 |
| 10 | 0.55 | 0.10 | 0.45 |

 =0.5mol/L��Z����ʼŨ��Ϊ0��7minʱ��Ӧ����ƽ�⣬ƽ��ʱCO�����ʵ���Ϊ0.55mol����CO��ƽ��Ũ��Ϊ
=0.5mol/L��Z����ʼŨ��Ϊ0��7minʱ��Ӧ����ƽ�⣬ƽ��ʱCO�����ʵ���Ϊ0.55mol����CO��ƽ��Ũ��Ϊ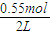 =0.275mol/L��ƽ��ʱZ�����ʵ���Ϊ0.45mol����Z��ƽ��Ũ��Ϊ
=0.275mol/L��ƽ��ʱZ�����ʵ���Ϊ0.45mol����Z��ƽ��Ũ��Ϊ =0.225mol/L���ݴ���ͼ��
=0.225mol/L���ݴ���ͼ��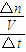 ����v��Z����
����v��Z����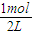 =0.5mol/L��Z����ʼŨ��Ϊ0��7minʱ��Ӧ����ƽ�⣬ƽ��ʱCO�����ʵ���Ϊ0.55mol����CO��ƽ��Ũ��Ϊ
=0.5mol/L��Z����ʼŨ��Ϊ0��7minʱ��Ӧ����ƽ�⣬ƽ��ʱCO�����ʵ���Ϊ0.55mol����CO��ƽ��Ũ��Ϊ =0.275mol/L��ƽ��ʱZ�����ʵ���Ϊ0.45mol����Z��ƽ��Ũ��Ϊ
=0.275mol/L��ƽ��ʱZ�����ʵ���Ϊ0.45mol����Z��ƽ��Ũ��Ϊ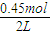 =0.225mol/L����CO��Z�����ʵ�����Ũ�ȣ�c����ʱ�䣨t���仯������Ϊ��
=0.225mol/L����CO��Z�����ʵ�����Ũ�ȣ�c����ʱ�䣨t���仯������Ϊ�� ��
��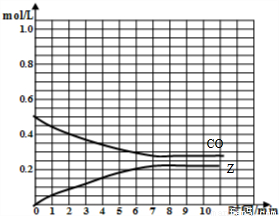 ��
�� =0.025 mol/��L?min����
=0.025 mol/��L?min����

 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij�¶�ʱ����2L�ܱ���������̬����X��Y��Ӧ������̬����Z�����ǵ����ʵ�����ʱ��ı仯�����ʾ��
ij�¶�ʱ����2L�ܱ���������̬����X��Y��Ӧ������̬����Z�����ǵ����ʵ�����ʱ��ı仯�����ʾ��| t/min | X/mol | Y/mol | Z/mol |
| 0 | 1.00 | 1.00 | 0.00 |
| 1 | 0.90 | 0.80 | 0.20 |
| 3 | 0.75 | 0.50 | 0.50 |
| 5 | 0.65 | 0.30 | 0.70 |
| 9 | 0.55 | 0.10 | 0.90 |
| 10 | 0.55 | 0.10 | 0.90 |
 2Z
2Z 2Z
2Z�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij�¶�ʱ����2L�ܱ������У�A��B��C�������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ����ͼ�����ݷ�����
ij�¶�ʱ����2L�ܱ������У�A��B��C�������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ����ͼ�����ݷ����� 2C��g��
2C��g�� 2C��g��
2C��g���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| t/min | X/mol | Y/mol | Z/mol |
| 0 | 1.00 | 1.00 | 0.00 |
| 1 | 0.90 | 0.80 | 0.20 |
| 3 | 0.75 | 0.50 | 0.50 |
| 5 | 0.65 | 0.30 | 0.70 |
| 9 | 0.55 | 0.10 | 0.90 |
| 10 | 0.55 | 0.10 | 0.90 |
| 14 | 0.55 | 0.10 | 0.90 |

 2Z
2Z 2Z
2Z�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
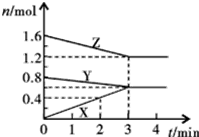 ij�¶�ʱ����2L�ܱ������У�X��Y��Z������̬���ʵ����ʵ�����ʱ��仯
ij�¶�ʱ����2L�ܱ������У�X��Y��Z������̬���ʵ����ʵ�����ʱ��仯�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com