
| A�� | 2�� | B�� | 3�� | C�� | 4�� | D�� | 5�� |
���� �پ�Ԫ�صķǽ�����ǿ���жϣ��ھݸ����ӵ��������жϣ��۾��������͵�����жϣ��ܶ�Ӧ����Խǿ����������ӵõ����ӵ�����Խ�����ݾ���Է���������С�жϣ���ɢ����ֱ��������Һ�����壾��Һ����������ǿ�ᣬ���ᡢ̼�ᡢ������Ϊ���ᣬ��������Ա�̼��ǿ������������Ը�����
��� �⣺��ͬ����������ҷǽ�������ǿ���ǽ�����Խǿ���⻯��Խ�ȶ��������ȶ��ԣ�HF��H2O��NH3���ʢ���ȷ��
�ھ�����ͬ�Ų������ӣ�ԭ������������Ӱ뾶С�������Ӱ뾶��F-��Na+��Mg2+���ʢڴ���
��CH4��CH3-������10�����ӣ��ʢ۴���
������ΪH2CO3��HCO3-��H2O����Ӧ����Խǿ����������ӵõ����ӵ�����Խ������������������OH-��CO32-��HCO3-���ʢ���ȷ��
��NH3����������е���ߣ�PH3��AsH3�������������Է�������Խ�е�Խ�ߣ����۷е㣺NH3��AsH3��PH3���ʢݴ���
��ɢ����ֱ��������Һ�����壾��Һ����Fe��OH��3����Һ��Fe��OH��3���壾FeCl3��Һ���ʢ���ȷ��
��������ǿ�ᣬ���ᡢ̼�ᡢ������Ϊ���ᣬ��������Ա�̼��ǿ������������Ը����������ԣ�HNO3��CH3COOH��H2CO3��HClO���ʢߴ���
��ѡB��
���� ���⿼���������ʵıȽϡ�Ԫ�������ɵ�Ӧ�ã��漰���뾶�Ƚϡ��������������ʡ���ɢϵ���������ʵȣ��ѶȲ���Ҫע�����֪ʶ�Ļ��ۣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
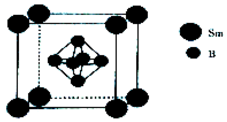 ���仯�����ڹ�ũҵ������Ӧ�ù㷺��
���仯�����ڹ�ũҵ������Ӧ�ù㷺���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ��ѧ���� | ʵ��Ӧ�� |
| A | Na��K���ǻ��ý��� | �ƼغϽ�����ԭ�ӷ�Ӧ�ѵ��ȼ� |
| B | H2O2��ʹ�����ʱ��� | ҽ���������˿����� |
| C | CH2=CH2����H2O�����ӳɷ�Ӧ | ��ϩ������ʵ�Ĵ���� |
| D | NH3���л�ԭ�� | ��ʳƷ�ӹ�������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ClO2�����������ˮ�У�ClO2��Ũ��Ӧ��0.10��0.80mg/L֮�䣮�õ��������ˮ��C1O2Ũ�ȵ�ʵ�鲽�����£�
��ClO2�����������ˮ�У�ClO2��Ũ��Ӧ��0.10��0.80mg/L֮�䣮�õ��������ˮ��C1O2Ũ�ȵ�ʵ�鲽�����£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 144.0gCuSO4 | B�� | 144.0gCuSO4•5H2O | ||
| C�� | 255.0gCuSO4•5H2O | D�� | 250.0gCuSO4•5H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1mol/L�Ȼ�ͭ��Һ�е�Cu2+��С��NA | |
| B�� | 28 g����ϩ���е�̼ԭ����Ϊ2NA | |
| C�� | ��״���£�22.4 L Cl2��ˮ��Ӧת�Ƶĵ�����ΪNA | |
| D�� | ���Ե缫���ʳ��ˮ������·��ͨ��2NA���ӵ�������������������22.4L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����BaSO3�������ݳ�SO3���� | |
| B�� | ����BaSO4�������ݳ�SO2���� | |
| C�� | ����BaSO4��BaSO3�������������ݳ� | |
| D�� | �������ɣ��ݳ�SO3��SO2���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com