
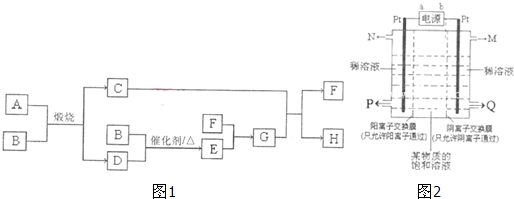
| 5 |
| 160 |
| 8 |
| 64 |
| 5 |
| 160 |
| 8 |
| 64 |
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��| 5 |
| 160 |
| 8 |
| 64 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����������������������Һ��2Al+6OH-�T2[Al��OH��4]-+3H2�� |
| B��̼�������Һ�м�������������Һ��HCO3-+OH-�TCO32-+H2O |
| C�����������������ռ���Һ��Ӧ��SiO2+2OH-�TSiO32-+H2O |
| D������μӵ�����������Һ�У�H++OH-�TH2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����Ũ�ȵ�NaClO��NaHCO3�����Һ�У�c��HClO��+c��ClO-��=c��HCO3-��+c��H2CO3�� |
| B��pH=4.5������֭��c��H+����pH=6.5�ķ���֭��c��H+����100�� |
| C��100mL pH=3��HA��Һ��HB��Һ�ֱ���������п��Ӧ��HA��Һ�ų��������϶࣬˵��HA�����Ա�HB��ǿ |
| D��pH=5.5��CH3COOH��CH3COONa�����Һ�У�c��Na+����c��CH3COO-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢� | B���ڢۢ� | C���٢� | D���ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢� | B���ڢ� | C���٢� | D���ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
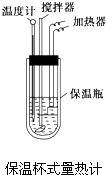 �����ȼ��н�100mL 0.50mol?L-1��CH3COOH��Һ��100mL 0.55mol?L-1��NaOH��Һ��ϣ��¶ȴ�298.0K������300.7K����֪���ȼƵ����ݳ��������ȼƸ�����ÿ����1K����Ҫ����������150.5J?K-1����Һ�ܶȾ�Ϊ1g?mL-1��������Һ�ı�����c=4.184J?��g?K��-1��
�����ȼ��н�100mL 0.50mol?L-1��CH3COOH��Һ��100mL 0.55mol?L-1��NaOH��Һ��ϣ��¶ȴ�298.0K������300.7K����֪���ȼƵ����ݳ��������ȼƸ�����ÿ����1K����Ҫ����������150.5J?K-1����Һ�ܶȾ�Ϊ1g?mL-1��������Һ�ı�����c=4.184J?��g?K��-1���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����NaAlO2��Һ��ͨ������CO2��AlO2-+CO2+2H2O�TAl��OH��3��+HCO3- |
| B�����ˮ�еμӱ��͵��Ȼ�����Һ��Fe3++3H2O�TFe��OH��3��+3H+ |
| C����̼���Ⱶ��Һ�м������������������Һ��Ba2++2HCO3-+2OH-�TBaCO3��+CO32-+2H2O |
| D����FeCl2��Һ�м�������K3[Fe��CN��6]��Һ��4Fe2++2[Fe��CN��6]4-�TFe4[Fe��CN��6]2�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com