
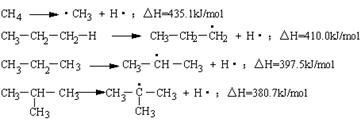
CH4![]() ��CH3+H������H=4351 kJ��mol-1
��CH3+H������H=4351 kJ��mol-1
CH3��CH2��CH2��H![]() CH3��CH2��
CH3��CH2��![]() H2+H������H=4100 kJ��mol-1
H2+H������H=4100 kJ��mol-1
CH3��CH2��CH3![]() CH3��
CH3��![]() H��CH3+H������H=3975 kJ��mol-1
H��CH3+H������H=3975 kJ��mol-1
 ����H=3807 kJ��mol-1
����H=3807 kJ��mol-1
���ṩ�����ж�����˵������ȷ����( )
A.�������С��C��H�����ѣ���ԭ��Խ�ױ�ȡ��
B.�ڹ��������±�������������ȡ����ӦCH3CH2CH2Cl��CH3CHClCH3���ʵ���֮��Ϊ1��1
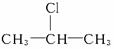
C.�춡������������ȡ����Ӧ��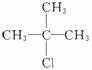 ռһ�ȴ�������ʵ�����������10%
ռһ�ȴ�������ʵ�����������10%
D.C2H6��C��H������ܽ���4100��4351 kJ��mol-1

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2007������ѧ������ѧÿ��һ�� (2) ���ͣ�013
|
����һ�������ļ�ʱ�����ĵ�������Ϊ����ܣ��ṩ����������C��H��������ܣ�
�����ṩ�����ж�����˵������ȷ���� | |
| [����] | |
A�� |
�������С��C��H�����ѣ���ԭ��Խ�ױ�ȡ�� |
B�� |
�ڹ��������±�������������ȡ����ӦCH3CH2CH2Cl��CH3CHClCH3�����ʵ���֮��Ϊ1��1 |
C�� |
�춡������������ȡ����Ӧ��(CH3)3CClռһ�ȴ�������ʵ�����������10�� |
D�� |
C2H6��C��H������ܽ���410.0kJ/mol~435.1kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ��ͤ����ѧ2007���������ѧ��ʱ��ϰһ ���ͣ�013
|
����һ�������ļ�ʱ�����ĵ�������Ϊ����ܣ��ṩ����������C��H��������ܣ�
�����ṩ�����ж�����˵������ȷ���� | |
| [����] | |
A�� |
�������С��C��H�����ѣ���ԭ��Խ�ױ�ȡ�� |
B�� |
�ڹ��������±���������ȡ����Ӧ����CH3CH2CH2Cl��CH3CHClCH3�����ʵ���֮��Ϊ1��1 |
C�� |
�춡������������ȡ����Ӧ��(CH3)3CClռһ�ȴ�������ʵ�����������10% |
D�� |
C2H6��C��H������ܽ���410.0kJ/mol~435.1kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������2007�������ҵ���ۺϲ��Ի�ѧ���� ���ͣ�013
|
����һ�������ļ�ʱ�����ĵ�������Ϊ����ܣ��ṩ����������C��H��������ܣ�
�����ṩ�����ж�����˵������ȷ���� | |
| [����] | |
A�� |
�������С��C��H�����ѣ���ԭ��Խ�ױ�ȡ�� |
B�� |
�ڹ��������±�������������ȡ����ӦCH3CH2CH2Cl��CH3CHClCH3�����ʵ���֮��Ϊ1��1 |
C�� |
�춡������������ȡ����Ӧ��(CH3)3CClռһ�ȴ�������ʵ�����������1/5 |
D�� |
C2H6��C��H������ܽ���410.0kJ/mol~435.1kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һ�������ļ�ʱ�����ĵ�������Ϊ����ܣ���������������C��H���������
�����жϲ���ȷ���� �� ��
A���������С��C��H�����ѣ���ԭ��Խ�ױ�ȡ��
B���ڹ��������±�������������ȡ����ӦCH3CH2CH2Cl��CH3CHClCH3���ʵ���֮��Ϊ1��1
C���춡������������ȡ����Ӧ��![]() ռһ�ȴ�������ʵ�����������10%
ռһ�ȴ�������ʵ�����������10%
D��C2H6��C��H������ܽ���410.0kJ/mol��435.1kJ/mol��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com