
|
ĻĀĶ¼±ķŹ¾µÄŹĒÄŃČÜĒāŃõ»ÆĪļŌŚ²»Ķ¬pHĻĀµÄČܽā¶Č(S/mol”¤L£1)£¬ĻĀĮŠĖµ·ØÖŠÕżČ·µÄŹĒ
| |
| [””””] | |
A£® |
pH£½3Ź±ČÜŅŗÖŠĢśŌŖĖŲµÄÖ÷ŅŖ“ęŌŚŠĪŹ½ŹĒFe3+ |
B£® |
ČōNi(NO3)2ČÜŅŗÖŠŗ¬ÓŠÉŁĮæµÄCo2+ŌÓÖŹ£¬æÉĶعżµ÷½ŚČÜŅŗpHµÄ·½·ØĄ“³żČ„ |
C£® |
Čō·ÖĄėČÜŅŗÖŠµÄFe3+ŗĶCu2+£¬æɵ÷½ŚČÜŅŗµÄpHŌŚ4×óÓŅ |
D£® |
ČōŌŚŗ¬ÓŠCu2+ŗĶNi2+µÄČÜŅŗÖŠ¼ÓČėÉÕ¼ī£¬Ni(OH)2ÓÅĻČ³Įµķ |
 Š”ѧ¶į¹ŚAB¾ķĻµĮŠ“š°ø
Š”ѧ¶į¹ŚAB¾ķĻµĮŠ“š°ø ABCæ¼ĶõČ«ÓžķĻµĮŠ“š°ø
ABCæ¼ĶõČ«ÓžķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013ÄźĘÕĶØøßµČѧŠ£ÕŠÉśĶ³Ņ»æ¼ŹŌ(½ĖÕ¾ķ)»ÆѧŹŌĢā ĢāŠĶ£ŗ022
Į׏ĒµŲæĒÖŠŗ¬Įæ½ĻĪŖ·įø»µÄ·Ē½šŹōŌŖĖŲ£¬Ö÷ŅŖŅŌÄŃČÜÓŚĖ®µÄĮ×ĖįŃĪČēCa3(PO4)2µČŠĪŹ½“ęŌŚ£®ĖüµÄµ„ÖŹŗĶ»ÆŗĻĪļŌŚ¹¤Å©ŅµÉś²śÖŠÓŠ×ÅÖŲŅŖµÄÓ¦ÓĆ£®
(1)°×Į×(P4)æÉÓÉCa3(PO4)2”¢½¹ĢæŗĶSiO2ŌŚŅ»¶ØĢõ¼žĻĀ·“Ó¦»ńµĆ£®Ļą¹ŲČČ»Æѧ·½³ĢŹ½ČēĻĀ£ŗ2Ca3(PO4)2(s)£«10C(s)![]() 6CaO(s)£«P4(s)£«10CO(g)””¦¤H1£½£«3359.26 kJ”¤mol£1
6CaO(s)£«P4(s)£«10CO(g)””¦¤H1£½£«3359.26 kJ”¤mol£1
CaO(s)£«SiO2(s)![]() CaSiO3(s)””¦¤H2£½£89.61 kJ”¤mol£1
CaSiO3(s)””¦¤H2£½£89.61 kJ”¤mol£1
2Ca3(PO4)2(s)£«6SiO2(s)£«10C(s)![]() 6CaSiO3(s)£«P4(s)£«10CO(g)””¦¤H3Ōņ¦¤H3£½________kJ”¤mol£1£®
6CaSiO3(s)£«P4(s)£«10CO(g)””¦¤H3Ōņ¦¤H3£½________kJ”¤mol£1£®
(2)°×Į×ÖŠ¶¾ŗóæÉÓĆCuSO4ČÜŅŗ½ā¶¾£¬½ā¶¾ŌĄķæÉÓĆĻĀĮŠ»Æѧ·½³ĢŹ½±ķŹ¾£ŗ
11P4£«60CuSO4£«96H2O![]() 20Cu3P£«24H3PO4£«60H2SO4
20Cu3P£«24H3PO4£«60H2SO4
60 mol””CuSO4ÄÜŃõ»Æ°×Į×µÄĪļÖŹµÄĮæŹĒ________£®
(3)Į×µÄÖŲŅŖ»ÆŗĻĪļNaH2PO4”¢Na2HPO4ŗĶNa3PO4æÉĶعżH3PO4ÓėNaOHČÜŅŗ·“Ó¦»ńµĆ£¬ŗ¬Į×ø÷ĪļÖֵķֲ¼·ÖŹż(Ę½ŗāŹ±Ä³ĪļÖÖµÄÅضČÕ¼ø÷ĪļÖÖÅضČÖ®ŗĶµÄ·ÖŹż)ÓėpHµÄ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£®
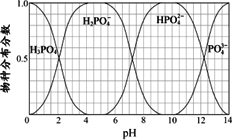
¢ŁĪŖ»ńµĆ¾”æÉÄÜ“æµÄNaH2PO4£¬pHÓ¦æŲÖĘŌŚ________£»pH£½8Ź±£¬ČÜŅŗÖŠÖ÷ŅŖŗ¬Į×ĪļÖÖÅØ¶Č“óŠ”¹ŲĻµĪŖ________£®
¢ŚNa2HPO4ČÜŅŗĻŌ¼īŠŌ£¬ČōĻņČÜŅŗÖŠ¼ÓČė×ćĮæµÄCaCl2ČÜŅŗ£¬ČÜŅŗŌņĻŌĖįŠŌ£¬ĘäŌŅņŹĒ________(ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾)£®
(4)Į׵ĻÆŗĻĪļČżĀČŃõĮ×(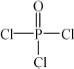 )Óė¼¾ĪģĖÄ“¼(
)Óė¼¾ĪģĖÄ“¼( )ŅŌĪļÖŹµÄĮæÖ®±Č2”Ć1·“Ó¦Ź±£¬æÉ»ńµĆŅ»ÖÖŠĀŠĶ×čČ¼¼ĮÖŠ¼äĢåX£¬²¢ŹĶ·Å³öŅ»ÖÖĖįŠŌĘųĢ壮¼¾ĪģĖÄ“¼ÓėXµÄŗĖ“Ź²ÕńĒāĘ×ČēĻĀĶ¼ĖłŹ¾£®
)ŅŌĪļÖŹµÄĮæÖ®±Č2”Ć1·“Ó¦Ź±£¬æÉ»ńµĆŅ»ÖÖŠĀŠĶ×čČ¼¼ĮÖŠ¼äĢåX£¬²¢ŹĶ·Å³öŅ»ÖÖĖįŠŌĘųĢ壮¼¾ĪģĖÄ“¼ÓėXµÄŗĖ“Ź²ÕńĒāĘ×ČēĻĀĶ¼ĖłŹ¾£®
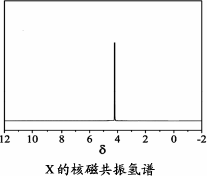
¢ŁĖįŠŌĘųĢåŹĒ________(Ģī»ÆѧŹ½)£®
¢ŚXµÄ½į¹¹¼ņŹ½ĪŖ________£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013ğȫ¹śĘÕĶØøßµČѧŠ£ÕŠÉśĶ³Ņ»æ¼ŹŌ»Æѧ£Ø½ĖÕ¾ķ“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
Į׏ĒµŲæĒÖŠŗ¬Įæ½ĻĪŖ·įø»µÄ·Ē½šŹōŌŖĖŲ£¬Ö÷ŅŖŅŌÄŃČÜÓŚĖ®µÄĮ×ĖįŃĪČēCa3(PO4)2µČŠĪŹ½“ęŌŚ”£ĖüµÄµ„ÖŹŗĶ»ÆŗĻĪļŌŚ¹¤Å©ŅµÉś²śÖŠÓŠ×ÅÖŲŅŖµÄÓ¦ÓĆ”£
£Ø1£©°×Į×(P4)æÉÓÉCa3(PO4)2”¢½¹ĢæŗĶSiO2ŌŚŅ»¶ØĢõ¼žĻĀ·“Ó¦»ńµĆ”£Ļą¹ŲČČ»Æѧ·½³ĢŹ½ČēĻĀ£ŗ
2Ca3(PO4)2(s)+10C(s)="==" 6CaO(s)+P4(s)+10CO(g) ”÷H1 ="+3359.26" kJ”¤mol£1
CaO(s)+SiO2(s)="==" CaSiO3(s) ”÷H2 ="-89." 61 kJ”¤mol£1
2Ca3(PO4)2(s)+6SiO2(s)+10C(s)="==" 6CaSiO3(s)+P4(s)+10CO(g) ”÷H3
Ōņ”÷H3 = kJ”¤mol£1”£
£Ø2£©°×Į×ÖŠ¶¾ŗóæÉÓĆCuSO4ČÜŅŗ½ā¶¾£¬½ā¶¾ŌĄķæÉÓĆĻĀĮŠ»Æѧ·½³ĢŹ½±ķŹ¾£ŗ
11P 4+60CuSO4+96H2O="==" 20Cu3P+24H3PO4+60H2SO4
60molCuSO4ÄÜŃõ»Æ°×Į×µÄĪļÖŹµÄĮæŹĒ ”£
£Ø3£©Į×µÄÖŲŅŖ»ÆŗĻĪļNaH2PO4”¢Na2HPO4ŗĶNa3PO4æÉĶعżH3PO4ÓėNaOHČÜŅŗ·“Ó¦»ńµĆ£¬ŗ¬Į×ø÷ĪļÖֵķֲ¼·ÖŹż(Ę½ŗāŹ±Ä³ĪļÖÖµÄÅضČÕ¼ø÷ĪļÖÖÅضČÖ®ŗĶµÄ·ÖŹż)ÓėpH µÄ¹ŲĻµČēĶ¼ĖłŹ¾”£
¢ŁĪŖ»ńµĆ¾”æÉÄÜ“æµÄNaH2PO4£¬pHÓ¦æŲÖĘŌŚ £»pH=8Ź±£¬ČÜŅŗÖŠÖ÷ŅŖŗ¬Į×ĪļÖÖÅØ¶Č“óŠ”¹ŲĻµĪŖ ”£
¢ŚNa2HPO4ČÜŅŗĻŌ¼īŠŌ£¬ČōĻņČÜŅŗÖŠ¼ÓČė×ćĮæµÄCaCl2ČÜŅŗ£¬ČÜŅŗŌņĻŌĖįŠŌ£¬ĘäŌŅņŹĒ
(ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾)”£
£Ø4£©Į׵ĻÆŗĻĪļČżĀČŃõĮ×(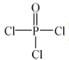 )Óė¼¾ĪģĖÄ“¼(
)Óė¼¾ĪģĖÄ“¼( )ŅŌĪļÖŹµÄĮæÖ®±Č2£ŗ1 ·“Ó¦Ź±£¬æÉ»ńµĆŅ»ÖÖŠĀŠĶ×čČ¼¼ĮÖŠ¼äĢåX£¬²¢ŹĶ·Å³öŅ»ÖÖĖįŠŌĘųĢ唣¼¾ĪģĖÄ“¼ÓėX µÄŗĖ“Ź²ÕńĒāĘ×ČēĻĀĶ¼ĖłŹ¾”£
)ŅŌĪļÖŹµÄĮæÖ®±Č2£ŗ1 ·“Ó¦Ź±£¬æÉ»ńµĆŅ»ÖÖŠĀŠĶ×čČ¼¼ĮÖŠ¼äĢåX£¬²¢ŹĶ·Å³öŅ»ÖÖĖįŠŌĘųĢ唣¼¾ĪģĖÄ“¼ÓėX µÄŗĖ“Ź²ÕńĒāĘ×ČēĻĀĶ¼ĖłŹ¾”£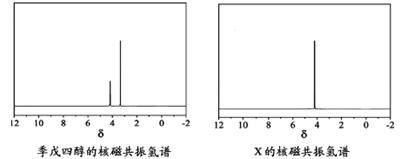
¢ŁĖįŠŌĘųĢåŹĒ (Ģī»ÆѧŹ½)”£
¢ŚXµÄ½į¹¹¼ņŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013ğȫ¹śĘÕĶØøßµČѧŠ£ÕŠÉśĶ³Ņ»æ¼ŹŌ»Æѧ£Ø½ĖÕ¾ķ½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
Į׏ĒµŲæĒÖŠŗ¬Įæ½ĻĪŖ·įø»µÄ·Ē½šŹōŌŖĖŲ£¬Ö÷ŅŖŅŌÄŃČÜÓŚĖ®µÄĮ×ĖįŃĪČēCa3(PO4)2µČŠĪŹ½“ęŌŚ”£ĖüµÄµ„ÖŹŗĶ»ÆŗĻĪļŌŚ¹¤Å©ŅµÉś²śÖŠÓŠ×ÅÖŲŅŖµÄÓ¦ÓĆ”£
£Ø1£©°×Į×(P4)æÉÓÉCa3(PO4)2”¢½¹ĢæŗĶSiO2ŌŚŅ»¶ØĢõ¼žĻĀ·“Ó¦»ńµĆ”£Ļą¹ŲČČ»Æѧ·½³ĢŹ½ČēĻĀ£ŗ
2Ca3(PO4)2(s)+10C(s)="==" 6CaO(s)+P4(s)+10CO(g) ”÷H1 ="+3359.26" kJ”¤mol£1
CaO(s)+SiO2(s)="==" CaSiO3(s) ”÷H2 ="-89." 61 kJ”¤mol£1
2Ca3(PO4)2(s)+6SiO2(s)+10C(s)="==" 6CaSiO3(s)+P4(s)+10CO(g) ”÷H3
Ōņ”÷H3 = kJ”¤mol£1”£
£Ø2£©°×Į×ÖŠ¶¾ŗóæÉÓĆCuSO4ČÜŅŗ½ā¶¾£¬½ā¶¾ŌĄķæÉÓĆĻĀĮŠ»Æѧ·½³ĢŹ½±ķŹ¾£ŗ
11P 4+60CuSO4+96H2O="==" 20Cu3P+24H3PO4+60H2SO4
60molCuSO4ÄÜŃõ»Æ°×Į×µÄĪļÖŹµÄĮæŹĒ ”£
£Ø3£©Į×µÄÖŲŅŖ»ÆŗĻĪļNaH2PO4”¢Na2HPO4ŗĶNa3PO4æÉĶعżH3PO4ÓėNaOHČÜŅŗ·“Ó¦»ńµĆ£¬ŗ¬Į×ø÷ĪļÖֵķֲ¼·ÖŹż(Ę½ŗāŹ±Ä³ĪļÖÖµÄÅضČÕ¼ø÷ĪļÖÖÅضČÖ®ŗĶµÄ·ÖŹż)ÓėpH µÄ¹ŲĻµČēĶ¼ĖłŹ¾”£
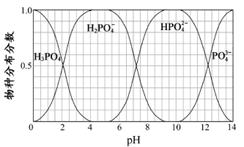
¢ŁĪŖ»ńµĆ¾”æÉÄÜ“æµÄNaH2PO4£¬pHÓ¦æŲÖĘŌŚ £»pH=8Ź±£¬ČÜŅŗÖŠÖ÷ŅŖŗ¬Į×ĪļÖÖÅØ¶Č“óŠ”¹ŲĻµĪŖ ”£
¢ŚNa2HPO4ČÜŅŗĻŌ¼īŠŌ£¬ČōĻņČÜŅŗÖŠ¼ÓČė×ćĮæµÄCaCl2ČÜŅŗ£¬ČÜŅŗŌņĻŌĖįŠŌ£¬ĘäŌŅņŹĒ
(ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾)”£
£Ø4£©Į׵ĻÆŗĻĪļČżĀČŃõĮ×(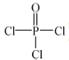 )Óė¼¾ĪģĖÄ“¼(
)Óė¼¾ĪģĖÄ“¼( )ŅŌĪļÖŹµÄĮæÖ®±Č2£ŗ1 ·“Ó¦Ź±£¬æÉ»ńµĆŅ»ÖÖŠĀŠĶ×čČ¼¼ĮÖŠ¼äĢåX£¬²¢ŹĶ·Å³öŅ»ÖÖĖįŠŌĘųĢ唣¼¾ĪģĖÄ“¼ÓėX µÄŗĖ“Ź²ÕńĒāĘ×ČēĻĀĶ¼ĖłŹ¾”£
)ŅŌĪļÖŹµÄĮæÖ®±Č2£ŗ1 ·“Ó¦Ź±£¬æÉ»ńµĆŅ»ÖÖŠĀŠĶ×čČ¼¼ĮÖŠ¼äĢåX£¬²¢ŹĶ·Å³öŅ»ÖÖĖįŠŌĘųĢ唣¼¾ĪģĖÄ“¼ÓėX µÄŗĖ“Ź²ÕńĒāĘ×ČēĻĀĶ¼ĖłŹ¾”£
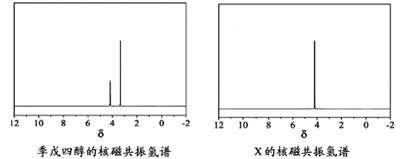
¢ŁĖįŠŌĘųĢåŹĒ (Ģī»ÆѧŹ½)”£
¢ŚXµÄ½į¹¹¼ņŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Į׏ĒµŲæĒÖŠŗ¬Įæ½ĻĪŖ·įø»µÄ·Ē½šŹōŌŖĖŲ£¬Ö÷ŅŖŅŌÄŃČÜÓŚĖ®µÄĮ×ĖįŃĪČēCa3(PO4)2µČŠĪŹ½“ęŌŚ”£ĖüµÄµ„ÖŹŗĶ»ÆŗĻĪļŌŚ¹¤Å©ŅµÉś²śÖŠÓŠ×ÅÖŲŅŖµÄÓ¦ÓĆ”£
£Ø1£©°×Į×(P4)æÉÓÉCa3(PO4)2”¢½¹ĢæŗĶSiO2 ŌŚŅ»¶ØĢõ¼žĻĀ·“Ó¦»ńµĆ”£Ļą¹ŲČČ»Æѧ·½³ĢŹ½ČēĻĀ£ŗ
2Ca3(PO4)2(s)+10C(s)=== 6CaO(s)+P4(s)+10CO(g) ”÷H1 =+3359.26 kJ”¤mol£1
CaO(s)+SiO2(s)=== CaSiO3(s) ”÷H2 =-89. 61 kJ”¤mol£1
2Ca3(PO4)2(s)+6SiO2(s)+10C(s)=== 6CaSiO3(s)+P4(s)+10CO(g) ”÷H3
Ōņ”÷H3 = kJ”¤mol£1”£
£Ø2£©°×Į×ÖŠ¶¾ŗóæÉÓĆCuSO4ČÜŅŗ½ā¶¾£¬½ā¶¾ŌĄķæÉÓĆĻĀĮŠ»Æѧ·½³ĢŹ½±ķŹ¾£ŗ
11P 4+60CuSO4+96H2O=== 20Cu3P+24H3PO4+60H2SO4
 60molCuSO4ÄÜŃõ»Æ°×Į×µÄĪļÖŹµÄĮæŹĒ ”£
60molCuSO4ÄÜŃõ»Æ°×Į×µÄĪļÖŹµÄĮæŹĒ ”£
£Ø3£©Į×µÄÖŲŅŖ»ÆŗĻĪļNaH2PO4”¢Na2HPO4ŗĶNa3PO4æÉĶعżH3PO4ÓėNaOHČÜŅŗ·“Ó¦»ńµĆ£¬ŗ¬Į×ø÷ĪļÖֵķֲ¼·ÖŹż(Ę½ŗāŹ±Ä³ĪļÖÖµÄÅضČÕ¼ø÷ĪļÖÖÅضČÖ®ŗĶµÄ·ÖŹż)ÓėpH µÄ¹ŲĻµČēÓŅĶ¼ĖłŹ¾”£
¢ŁĪŖ»ńµĆ¾”æÉÄÜ“æµÄNaH2PO4£¬pHÓ¦æŲÖĘŌŚ £»pH=8Ź±£¬ČÜŅŗÖŠÖ÷ŅŖŗ¬Į×ĪļÖÖÅØ¶Č“óŠ”¹ŲĻµĪŖ ”£
¢ŚNa2HPO4 ČÜŅŗĻŌ¼īŠŌ£¬ČōĻņČÜŅŗÖŠ¼ÓČė×ćĮæµÄCaCl2 ČÜŅŗ£¬ČÜŅŗŌņĻŌĖįŠŌ£¬ĘäŌŅņŹĒ
(ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾)”£
£Ø4£©Į׵ĻÆŗĻĪļČżĀČŃõĮ×( )Óė¼¾ĪģĖÄ“¼(
)Óė¼¾ĪģĖÄ“¼(![]() )ŅŌĪļÖŹµÄĮæÖ®±Č2£ŗ1 ·“Ó¦Ź±£¬æÉ»ńµĆŅ»ÖÖŠĀŠĶ×čČ¼¼ĮÖŠ¼äĢåX£¬²¢ŹĶ·Å³öŅ»ÖÖĖįŠŌĘųĢ唣¼¾ĪģĖÄ“¼ÓėX µÄŗĖ“Ź²ÕńĒāĘ×ČēĻĀĶ¼ĖłŹ¾”£
)ŅŌĪļÖŹµÄĮæÖ®±Č2£ŗ1 ·“Ó¦Ź±£¬æÉ»ńµĆŅ»ÖÖŠĀŠĶ×čČ¼¼ĮÖŠ¼äĢåX£¬²¢ŹĶ·Å³öŅ»ÖÖĖįŠŌĘųĢ唣¼¾ĪģĖÄ“¼ÓėX µÄŗĖ“Ź²ÕńĒāĘ×ČēĻĀĶ¼ĖłŹ¾”£

¢ŁĖįŠŌĘųĢåŹĒ (Ģī»ÆѧŹ½)”£
¢ŚXµÄ½į¹¹¼ņŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com