
��һƿ������Һ�����п��ܺ���NH4����K����Ba2����Al3����Fe3����I����NO3����CO32����SO42����AlO2����ȡ����Һ��������ʵ�飺
����pH��ֽ���飬��Һ��ǿ���ԣ�
��ȡ��Һ��������������CCl4������������ˮ����CCl4����Ϻ�ɫ��
����ȡ��Һ��������μ���NaOH��Һ��
a����Һ�����Ա�Ϊ���ԣ�b.��Һ����������c.������ȫ�ܽ⣻d.��������Һ��������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������
��ȡ�����۵õ��ļ�����Һ������Na2CO3��Һ���а�ɫ�������ɡ�
��������ʵ�����ش��������⡣
(1)�ɢٿ����ų�________�Ĵ��ڡ�
(2)�ɢڿ���֤��________�Ĵ��ڣ�ͬʱ�ų�________�Ĵ��ڣ�������____________��
(3)�ɢۿ���֤��________�Ĵ��ڣ�д��c��d���漰�Ļ�ѧ����ʽ�������ӷ�Ӧ�����������ʽ��ʾ��c__________��d______________��
(4)�ɢܿ����ų�________�Ĵ��ڣ�ͬʱ֤��________�Ĵ��ڡ�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������������棬��2011��5��1���𣬽�ֹ��������������ӹ������ƣ�CaO2����ʳƷ���Ӽ������жԹ������Ƶ������������
A��CaO2���������ԣ�����ۿ��ܾ�����������
B��CaO2��ˮ��Ӧʱ��ÿ����1 mol O2ת�Ƶ���2 mol
C��CaO2���������ӵĸ�����Ϊ2��1
D��CaO2��CO2��Ӧ�Ļ�ѧ����ʽΪ��2CaO2+2CO2 ��2Ca CO3+O2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ʵ�ˮ��Һ�зֱ��������ʯ��ˮ��ԭ��Һ�е������Ӻ������Ӷ����ٵ���
A.FeCl2 B.CuSO4 C. Na2CO3 D.Ba(NO3)2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��A��B��C��D��E���ֳ���������������±��е������γɵģ�
| ������ | K����Na����Cu2����Al3�� |
| ������ | SO42����HCO3����NO3����OH�� |
Ϊ�˼�������������ֱ��������ʵ�飬�����ǣ�
�ٽ���������ˮ��DΪ��ɫ��Һ��������Ϊ��ɫ��Һ��
�ڽ�E��Һ���뵽C��Һ�г��ְ�ɫ�����������μӣ������ܽ⣻
�۽�����ɫ��Ӧ��ֻ��B��CΪ��ɫ(����ɫ�ܲ���)��
���ڸ���Һ�м������ᱵ��Һ���ټ������ϡ���ᣬA�зų���ɫ���壬C��D�в�����ɫ������
�ݽ�B��D����Һ��ϣ�δ���������������ɡ�
��������ʵ����գ�
(1)д��B��C��D�Ļ�ѧʽ��B________��C________��D________��
(2)����1 mol A����Һ�뺬1 mol E����Һ��Ӧ�����ɣ����õ�һ�ֻ�����û�����Ļ�ѧʽΪ________��
(3)��A��Һ�м�����������ʯ��ˮ�������ӷ���ʽΪ______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪ij��Һ�п��ܺ���SO ��SO
��SO ������ijЩδ֪���ӣ�ijͬѧȡ������Һ������֧�Թ��У�Ȼ��ֱ��������ʵ�鲢�ó���Ӧ�Ľ��ۣ����к������� (����)
������ijЩδ֪���ӣ�ijͬѧȡ������Һ������֧�Թ��У�Ȼ��ֱ��������ʵ�鲢�ó���Ӧ�Ľ��ۣ����к������� (����)
��������BaCl2��Һ�õ���ɫ������Ȼ�����������ϡ���ᣬ��������ʧ��˵��ԭ��Һ��һ������SO
��������BaCl2��Һ�õ���ɫ������Ȼ�����������ϡ���ᣬ�����ܽⲢ�����̼�����ζ�����壬˵��ԭ��Һ��һ������SO
�����ȼ��������������ټ���BaCl2��Һ�õ���ɫ������˵��ԭ��Һ��һ����SO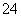
��������ɫ��Ӧʵ��ʱ������ʻ�ɫ��˵����Һ��һ����Na����������ȷ���Ƿ���K��
A���٢ڢۢ�  B���٢�
B���٢�
C���ڢۢ� D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ɫ��Һ�к���K����Cl����OH����SO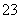 ��SO
��SO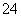 ��Ϊ������Һ��������ijЩ�����ӣ����õ��Լ��У����ᡢ���ᡢ��������Һ�����ᱵ��Һ����ˮ�ͷ�̪��Һ����������OH����ʵ�鷽��ʡ�ԣ��������������ӵĹ�������ͼ��ʾ��
��Ϊ������Һ��������ijЩ�����ӣ����õ��Լ��У����ᡢ���ᡢ��������Һ�����ᱵ��Һ����ˮ�ͷ�̪��Һ����������OH����ʵ�鷽��ʡ�ԣ��������������ӵĹ�������ͼ��ʾ��
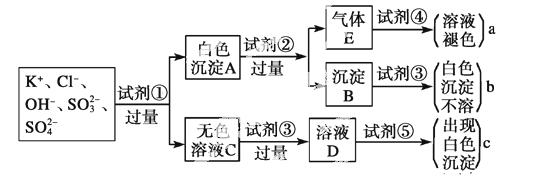
(1)ͼ���Լ��١������ʵĻ�ѧʽ�ֱ���
��________����________����________����__________��
��__________��
(2)ͼ������a��b��c��������������ӷֱ���
a________��b___ _____��c________��
_____��c________��
(3)��ɫ����A���Լ��ڷ�Ӧ�����ӷ���ʽ��_________________________________
________________________________________________________________________��
(4)��ɫ��ҺC���Լ��۵���ҪĿ����___________________________ __________��
__________��
(5)��ɫ����A�����Լ��۶������Լ��ڣ���ʵ���Ӱ����____________________��
(6)����Eͨ���Լ��ܷ�����Ӧ�����ӷ���ʽ��_______________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڼ�����Һ���ܴ�����������ҺΪ��ɫ������ (����)
A��K����MnO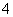 ��Na����Cl��
��Na����Cl��
B��K����Na����NO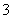 ��CO
��CO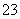
C��Na����H����NO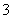 ��SO
��SO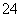
D��Fe3����Na����Cl����SO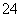
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£����и���������ָ����Һ��һ���ܴ���������ǣ� ��
A��ʹ���ȱ��ɫ����Һ��Mg2����K����SO42�� ��NO3��
B��ʹ��̪���ɫ����Һ��Na����Cu2����HCO3�� ��NO3��
C��0. 1 mol·L-1AgNO3 ��Һ��H����K����SO42�� ��I��
D��0. 1 mol·L-1NaAlO2 ��Һ: H����Na����Cl����SO42��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ�����ӵ���������ֵ������˵����ȷ����(����)
A��1 mol����(—NH2)�к���10NA������
B����״���£�2.24 L18O2�к���2NA������
C����״���£�22.4 L C8H18�к���25NA�����ۼ�
D���ڷ�ӦCaO��3C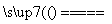 CaC2��CO���У�ÿ����1 mol CO��ת��3NA������
CaC2��CO���У�ÿ����1 mol CO��ת��3NA������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com