
ŅŃÖŖĮņĖį”¢°±Ė®µÄĆܶČÓėĖł¼ÓĖ®ĮæµÄ¹ŲĻµČēĶ¼ĖłŹ¾£¬ĻÖÓŠĮņĖįÓė°±Ė®ø÷Ņ»·Ż£¬Ēėøł¾Ż±ķÖŠŠÅĻ¢£¬»Ų“šĻĀĮŠĪŹĢā£ŗ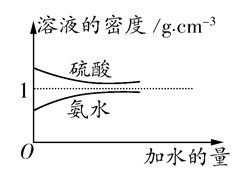
| | ČÜÖŹµÄĪļÖŹµÄĮæ ÅضČ(mol”¤L£1) | ČÜŅŗµÄĆܶČ(g”¤cm£3) |
| ĮņĖį | c1 | ¦Ń1 |
| °±Ė® | c2 | ¦Ń2 |
 c2 mol”¤L£1µÄ°±Ė®µČÖŹĮæ»ģŗĻ£¬ĖłµĆČÜŅŗµÄĆÜ¶Č (Ģī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£¬ĻĀĶ¬)¦Ń2 g”¤cm£3£¬ĖłµĆČÜŅŗµÄĪļÖŹµÄĮæÅضČ
c2 mol”¤L£1µÄ°±Ė®µČÖŹĮæ»ģŗĻ£¬ĖłµĆČÜŅŗµÄĆÜ¶Č (Ģī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£¬ĻĀĶ¬)¦Ń2 g”¤cm£3£¬ĖłµĆČÜŅŗµÄĪļÖŹµÄĮæÅØ¶Č 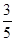 c2 mol”¤L£1(Éč»ģŗĻŗóČÜŅŗµÄĢå»ż±ä»ÆŗöĀŌ²»¼Ę)”£
c2 mol”¤L£1(Éč»ģŗĻŗóČÜŅŗµÄĢå»ż±ä»ÆŗöĀŌ²»¼Ę)”£  ŠĀĖ¼Ī¬¼ŁĘŚ×÷ŅµŹī¼Ł¼ŖĮÖ“óѧ³ö°ęÉēĻµĮŠ“š°ø
ŠĀĖ¼Ī¬¼ŁĘŚ×÷ŅµŹī¼Ł¼ŖĮÖ“óѧ³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
NA“ś±ķ°¢·ü¼ÓµĀĀŽ³£Źż£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø £©
| A£®±ź×¼×“æöĻĀ£¬22.4 LSO3ÖŠŗ¬ÓŠµÄ·Ö×ÓŹżĪŖNA |
| B£®100 mL 2.0 mol/LµÄŃĪĖįÓė“×ĖįČÜŅŗÖŠĒāĄė×Ó¾łĪŖ0.2NA |
| C£®0.1mol/LĻ”ĮņĖįÖŠŗ¬SO42-µÄŹżÄæĪŖ0.1NA |
| D£®±ź×¼×“æöĻĀ£¬11.2LNOŗĶ5.6LO2»ģŗĻŗó,·Ö×Ó×ÜŹżŠ”ÓŚ0.5NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¢ń£®°“ŅŖĒóĢīæÕ(ĆææÕ1·Ö)
¢ŁKOHµÄµē×ÓŹ½: ¢ŚNH4ClµÄµē×ÓŹ½£ŗ
¢ŪCO2µÄ½į¹¹Ź½: ¢ÜHClOµÄ½į¹¹Ź½:
¢ņ£®°“ŅŖĒóĢīæÕ(ĆææÕ2·Ö)
ėĀ£ØN2H4£©ÓÖ³ĘĮŖ°±£¬ŹĒŅ»ÖÖæÉČ¼ŠŌµÄŅŗĢ壬æÉÓĆ×÷»š¼żČ¼ĮĻ”£ŅŃÖŖŌŚ101kPa£Ø25”ꏱ£©Ź±£¬ŅŃÖŖ0£®5molŅŗĢ¬ėĀÓė×ćĮæŃõĘų·“Ó¦£¬Éś³ÉµŖĘųŗĶĖ®ÕōĘų£¬·Å³ö312 KJµÄČČĮ攣N2H4ĶźČ«Č¼ÉÕ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ŹĒ £»ÓÖŅŃÖŖH2O(l)==H2O(g)£»”÷H = +44kJ?mol-1£¬ÓÉ64gŅŗĢ¬ėĀÓėŃõĘų·“Ӧɜ³ÉŅŗĢ¬Ė®Ź±·Å³öµÄČČĮæŹĒ kJ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
³ōŃõ²ćŹĒµŲĒņÉśĆüµÄ±£»¤Éń£¬³ōŃõ±ČŃõĘų¾ßÓŠøüĒæµÄŃõ»ÆŠŌ”£ŹµŃéŹŅæɽ«ŃõĘųĶعżøßŃ¹·Åµē¹ÜĄ“ÖĘČ”³ōŃõ£ŗ3O2 2O3”£
2O3ӣ
(1)ČōŌŚÉĻŹö·“Ó¦ÖŠÓŠ30%µÄŃõĘų×Ŗ»ÆĪŖ³ōŃõ£¬ĖłµĆ»ģŗĻĘųµÄĘ½¾łÄ¦¶ūÖŹĮæĪŖ________g/mol(±£ĮōŅ»Ī»Š”Źż)”£
(2)½«8 LŃõĘųĶعż·Åµē¹Üŗ󣬻Öø“µ½Ōדæö£¬µĆµ½ĘųĢå6.5 L£¬ĘäÖŠ³ōŃõĪŖ________L”£
(3)ŹµŃéŹŅ½«ŃõĘųŗĶ³ōŃõµÄ»ģŗĻĘųĢå0.896 L”£(±ź×¼×“æö)ĶØČėŹ¢ÓŠ20.0 gĶ·ŪµÄ·“Ó¦Ę÷ÖŠ£¬³ä·Ö¼ÓČČŗ󣬷ŪÄ©µÄÖŹĮæ±äĪŖ21.6 g”£ŌņŌ»ģŗĻĘųÖŠ³ōŃõµÄĢå»ż·ÖŹżĪŖ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
µ„ÖŹ¹čŹĒŗÜÖŲŅŖµÄ¹¤Ņµ²śĘ·”£
£Ø1£©¹čÓĆÓŚŅ±Į¶Ć¾£¬Ņ²³Ę¹čČČ·ØĮ¶Ć¾”£øł¾ŻĻĀĮŠĢõ¼ž£ŗ
Mg£Øs£©£« 1/2O2£Øg£©£½ MgO£Øs£© ”÷H1£½£601£®8 kJ/mol
Mg£Øs£©£½ Mg£Øg£© ”÷H2£½+75 kJ/mol
Si£Øs£© £« O2£Øg£© £½ SiO2£Øs£© ”÷H3£½ £859£®4 kJ/mol
Ōņ2MgO£Øs£©£« Si£Øs£©£½ SiO2£Øs£©£« 2Mg£Øg£© ”÷H £½
Mg-NiOOHĖ®¼¤»īµē³ŲŹĒÓćĄ×µÄ³£ÓƵē³Ų£¬µē³Ų×Ü·“Ó¦ŹĒ£ŗMg+2NiOOH+2H2O=Mg(OH)2+ 2Ni(OH)2£¬Š“³öµē³ŲÕż¼«µÄµē¼«·“Ó¦Ź½ ”£
£Ø2£©Öʱø¶ą¾§¹č£Ø¹čµ„ÖŹµÄŅ»ÖÖ£©µÄø±²śĪļÖ÷ŅŖŹĒSiCl4£¬SiCl4¶Ō»·¾³ĪŪČ¾ŗÜ“ó£¬ÓöĖ®ĒæĮŅĖ®½ā£¬·Å³ö“óĮæµÄČČ”£ŃŠ¾æČĖŌ±ĄūÓĆSiCl4ŗĶ±µæó·Ū£ØÖ÷ŅŖ³É·ÖĪŖBaCO3£¬ĒŅŗ¬ÓŠFe3+”¢Mg2+µČĄė×Ó£©ÖʱøBaCl2”¤2H2OŗĶSiO2µČĪļÖŹ”£¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ
ŅŃÖŖ£ŗ 25”ę Ksp[Fe(OH)3]£½4£®0”Į10-38£¬ Ksp[Mg(OH)2]£½1£®8”Į10-11£»Ķس£ČĻĪŖ²ŠĮōŌŚČÜŅŗÖŠµÄĄė×ÓÅØ¶ČŠ”ÓŚ1”Į10-5mol/LŹ±£¬³Įµķ¾Ķ“ļĶźČ«”£»Ų“šĻĀĮŠĪŹĢā£ŗ
¢ŁSiCl4·¢ÉśĖ®½ā·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_______________________________________”£
¢ŚČō¼Ó±µæó·Ūµ÷½ŚpH=3Ź±£¬ČÜŅŗÖŠc(Fe3+)= ”£
¢ŪČōÓĆ10¶Öŗ¬78% BaCO3µÄ±µæó·Ū£¬×īÖÕµĆµ½8£®4¶ÖBaCl2”¤2H2O (M=244g/mol)£¬Ōņ²śĀŹĪŖ ”£
¢ÜĀĖŌüCÄÜ·Ö±šČÜÓŚÅØ¶Č¾łĪŖ3mol/LµÄ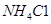 ČÜŅŗŗĶ
ČÜŅŗŗĶ ČÜŅŗ£ØÖŠŠŌ£©”£Ēė½įŗĻĘ½ŗāŌĄķŗĶ±ŲŅŖµÄĪÄ×Ö½āŹĶĀĖŌüCÄÜČÜÓŚ3mol/LµÄ
ČÜŅŗ£ØÖŠŠŌ£©”£Ēė½įŗĻĘ½ŗāŌĄķŗĶ±ŲŅŖµÄĪÄ×Ö½āŹĶĀĖŌüCÄÜČÜÓŚ3mol/LµÄ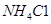 ČÜŅŗµÄŌŅņ______”£
ČÜŅŗµÄŌŅņ______”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÄĘŹĒ»īĘĆµÄ¼ī½šŹōŌŖĖŲ£¬ÄĘ¼°Ęä»ÆŗĻĪļŌŚÉś²śŗĶÉś»īÖŠÓŠ¹ć·ŗµÄÓ¦ÓĆ”£
Ķź³ÉĻĀĮŠ¼ĘĖć£ŗ
(1)µžµŖ»ÆÄĘ(NaN3)ŹÜײ»÷ĶźČ«·Ö½ā²śÉśÄĘŗĶµŖĘų£¬¹ŹæÉÓ¦ÓĆÓŚĘū³µ°²Č«ĘųÄŅ”£Čō²śÉś40.32 L(±ź×¼×“æöĻĀ)µŖĘų£¬ÖĮÉŁŠčŅŖµžµŖ»ÆÄĘ________g”£
(2)ÄĘ¼ŲŗĻ½šæÉŌŚŗĖ·“Ó¦¶ŃÖŠÓĆ×÷ČČ½»»»Ņŗ”£5.05 gÄĘ¼ŲŗĻ½šČÜÓŚ200 mLĖ®Éś³É0.075 molĒāĘų”£
¢Ł¼ĘĖćČÜŅŗÖŠĒāŃõøłĄė×ÓµÄĪļÖŹµÄĮæÅضČ(ŗöĀŌČÜŅŗĢå»ż±ä»Æ)”£
_____________________________________________________________
¢Ś¼ĘĖć²¢Č·¶ØøĆÄĘ¼ŲŗĻ½šµÄ»ÆѧŹ½”£
_____________________________________________________________
(3)ĒāŃõ»ÆÄĘČÜŅŗ“¦ĄķĀĮĶĮæó²¢¹żĀĖ£¬µĆµ½ŗ¬ĀĮĖįÄʵÄČÜŅŗ”£ĻņøĆČÜŅŗÖŠĶØČė¶žŃõ»ÆĢ¼£¬ÓŠĻĀĮŠ·“Ó¦£ŗ
2NaAlO2£«3H2O£«CO2=2Al(OH)3”ż£«Na2CO3
ŅŃÖŖĶØČė¶žŃõ»ÆĢ¼336 L(±ź×¼×“æöĻĀ)£¬Éś³É24 mol Al(OH)3ŗĶ15 mol Na2CO3£¬ČōĶØČėČÜŅŗµÄ¶žŃõ»ÆĢ¼ĪŖ112 L(±ź×¼×“æöĻĀ)£¬¼ĘĖćÉś³ÉµÄAl(OH)3ŗĶNa2CO3µÄĪļÖŹµÄĮæÖ®±Č”£
_________________________________________________________________
(4)³£ĪĀĻĀ£¬³ĘČ”²»Ķ¬ĒāŃõ»ÆÄĘѳʷČÜÓŚĖ®£¬¼ÓŃĪĖįÖŠŗĶÖĮpH£½7£¬Č»ŗó½«ČÜŅŗÕōøɵĆĀČ»ÆÄĘ¾§Ģ壬Õōøɹż³ĢÖŠ²śĘ·ĪŽĖšŹ§”£
| | ĒāŃõ»ÆÄĘÖŹĮæ(g) | ĀČ»ÆÄĘÖŹĮæ(g) |
| ¢Ł | 2.40 | 3.51 |
| ¢Ś | 2.32 | 2.34 |
| ¢Ū | 3.48 | 3.51 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŃĪÄąŹĒĀČ¼ī¹¤ŅµÖŠµÄ·ĻŌü£¬Ö÷ŅŖ³É·ÖŹĒĆ¾µÄ¹čĖįŃĪŗĶĢ¼ĖįŃĪ(ŗ¬ÉŁĮæĢś”¢ĀĮ”¢øʵÄŃĪ)”£ŹµŃéŹŅŅŌŃĪÄąĪŖŌĮĻÖĘČ”MgSO4”¤7H2OµÄŹµŃé¹ż³ĢČēĻĀ£ŗ
¢ŁĖ®£¬µ÷³ÉÄą½¬¢ŚĻ”ĮņĖįµ÷pHĪŖ1”«2¢ŪÖ󷊢ܹżĀĖØD”śØD”ś²śĘ·
ŅŃÖŖ£ŗ¢ŁŹŅĪĀĻĀKsp[Mg(OH)2]£½6.0”Į10£12”£¢ŚŌŚČÜŅŗÖŠ£¬Fe2£«”¢Fe3£«”¢Al3£«“ÓæŖŹ¼³Įµķµ½³ĮµķĶźČ«µÄpH·¶Ī§ŅĄ“ĪĪŖ7.1”«9.6”¢2.0”«3.7”¢3.1”«4.7”£¢ŪČżÖÖ»ÆŗĻĪļµÄČܽā¶Č(S)ĖęĪĀ¶Č±ä»ÆµÄĒśĻßČēĶ¼ĖłŹ¾”£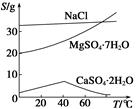
£Ø1£©ŌŚŃĪÄąÖŠ¼ÓČėĻ”ĮņĖįµ÷pHĪŖ1”«2ŅŌ¼°Ö󷊵ÄÄæµÄŹĒ_____________________”£
£Ø2£©ČōŹŅĪĀĻĀµÄČÜŅŗÖŠMg2£«µÄÅضČĪŖ6.0 mol”¤L£1£¬ŌņČÜŅŗpH”Ż________²ÅæÉÄܲśÉśMg(OH)2³Įµķ”£
£Ø3£©ÓÉĀĖŅŗ¢ńµ½ĀĖŅŗ¢ņŠčĻČ¼ÓČėNaClOµ÷ČÜŅŗpHŌ¼ĪŖ5£¬ŌŁ³ĆČČ¹żĀĖ£¬Ōņ³ĆČČ¹żĀĖµÄÄæµÄŹĒ__________________£¬ĀĖŌüµÄÖ÷ŅŖ³É·ÖŹĒ______________________”£
£Ø4£©“ÓĀĖŅŗ¢ņÖŠ»ńµĆMgSO4”¤7H2O¾§ĢåµÄŹµŃé²½ÖčŅĄ“ĪĪŖ¢ŁĻņĀĖŅŗ¢ņÖŠ¼ÓČė______________£»¢Ś¹żĀĖ£¬µĆ³Įµķ£»¢Ū________________£»¢ÜÕō·¢ÅØĖõ£¬½µĪĀ½į¾§£»¢Ż¹żĀĖ”¢Ļ“µÓµĆ²śĘ·”£
£Ø5£©Čō»ńµĆµÄMgSO4”¤7H2OµÄÖŹĮæĪŖ24.6 g£¬ŌņøĆŃĪÄąÖŠĆ¾[ŅŌMg(OH)2¼Ę]µÄ°Ł·Öŗ¬ĮæŌ¼ĪŖ________(MgSO4”¤7H2OµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ246)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¶ž¼×Ėį¼ŲŹĒŅ»ÖÖ°×É«¾§Ģ壬ÉĢĘ·ĆūĪŖø„Ć×(Formil)£¬ŹĒŅ»ÖÖÄÜĢę“śæ¹ÉśĖŲµÄ“ŁÉś³¤¼Į£¬»ÆѧŹ½ĪŖKH(HCOO)2£¬¾ßÓŠĪüŹŖŠŌ£¬Ņ×ČÜÓŚĖ®”£
¶ž¼×Ėį¼ŲµÄÉś²ś¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ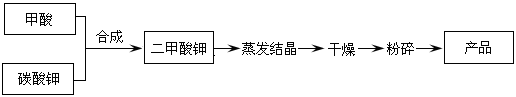
£Ø1£©Š“³ö¼×ĖįŗĶĢ¼Ėį¼ŲÉś²ś¶ž¼×Ėį¼ŲµÄ»Æѧ·½³ĢŹ½£ŗ ”£
£Ø2£©²ā¶Øijø„ĆײśĘ·“æ¶ČµÄŹµŃé·½·ØČēĻĀ£ŗ
³ĘČ”øĆø„ĆײśĘ·2.5g£¬½«ĘäČ«²æČܽāŌŚĖ®ÖŠ£¬ÅäÖĘ³É250mLĪ“ÖŖÅØ¶ČµÄČÜŅŗ£¬Č”³ö25.00mLӌ׶ŠĪĘæÖŠ£¬ŌŁµĪ¼Ó2”«3µĪÖøŹ¾¼Į£¬ÓĆ0.10mol”¤L£1µÄNaOHČÜŅŗµĪ¶ØÖĮÖÕµć£¬ĻūŗÄNaOHČÜŅŗµÄĢå»żĪŖ18.50mL”£
¢Ł¼ÓČėµÄÖøŹ¾¼ĮŹĒ £ØŃ”Ģī”°¼×»ł³Č”±”¢”°ŹÆČļ”±»ņ”°·ÓĢŖ”±£©£¬ÓĆNaOHČÜŅŗµĪ¶ØÖĮÖÕµćµÄĻÖĻóŹĒ ”£
¢ŚÉĻŹöø„ĆײśĘ·ÖŠ¶ž¼×Ėį¼ŲµÄÖŹĮæ·ÖŹżĪŖ ”££ØŠ“³ö¼ĘĖć¹ż³Ģ£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĒāĘųŹĒĒå½ąµÄÄÜŌ“£¬Ņ²ŹĒÖŲŅŖµÄ»Æ¹¤ŌĮĻ”£
£Ø1£©ŅŌH2ĪŖŌĮĻÖĘČ”°±Ęų½ų¶ųŗĻ³ÉCO(NH2)2µÄ·“Ó¦ČēĻĀ£ŗ
N2(g)£«3H2(g)£½2NH3(g) ”÷H£½ØD92.40 kJ”¤mol£1
2NH3(g)£«CO2(g)£½NH2CO2NH4(s) ”÷H£½ØD159.47 kJ”¤mol£1
NH2CO2NH4(s)£½CO(NH2)2(s)£«H2O(l) ”÷H£½£«72.49 kJ”¤mol£1
ŌņN2(g)”¢H2(g)ÓėCO2(g)·“Ӧɜ³ÉCO(NH2)2(s)ŗĶH2O(l)µÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø2£©ÓƱūĶéŗĶĖ®ĪŖŌĮĻŌŚµē“ß»ÆĻĀÖĘĒāĘų£¬Ķ¬Ź±µĆµ½Ņ»ÖÖŗ¬ÓŠČżŌŖ»·µÄ»·Ńõ»ÆŗĻĪļA£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£øĆ·“Ó¦Ņ²æÉÉś³ÉAµÄĶ¬·ÖŅģ¹¹Ģå”Ŗ”ŖĮķŅ»ÖÖ»·Ńõ»ÆŗĻĪļB£¬BµÄŗĖ“Ź²ÕńĒāĘ×ĪŖĻĀĶ¼ÖŠµÄ £ØĢī”°a”±»ņ”°b”±£©”£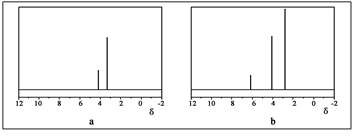
£Ø3£©ŅŃÖŖµžµŖĖį£ØHN3£©²»ĪČ¶Ø£¬Ķ¬Ź±Ņ²ÄÜÓė»īĘĆ½šŹō·“Ó¦£¬·“Ó¦·½³ĢŹ½ĪŖ£ŗ
2HN3£½3N2”ü£«H2”ü
2HN3£«Zn£½Zn(N3)2£«H2”ü
2 mol HN3ÓėŅ»¶ØĮæZnĶźČ«·“Ó¦£¬ŌŚ±ź×¼×“æöĻĀÉś³É67.2 LĘųĢ壬ĘäÖŠN2µÄĪļÖŹµÄĮæĪŖ ”£
£Ø4£©ŅŃÖŖH2SøßĪĀČČ·Ö½āÖĘH2µÄ·“Ó¦ĪŖ£ŗH2S(g) H2(g)£«1/2S2(g) ŌŚŗćČŻĆܱÕČŻĘ÷ÖŠ£¬æŲÖĘ²»Ķ¬ĪĀ¶Č½ųŠŠH2SµÄ·Ö½āŹµŃé£ŗŅŌH2SµÄĘšŹ¼ÅØ¶Č¾łĪŖc mol”¤L£1²ā¶ØH2SµÄ×Ŗ»ÆĀŹ£¬½į¹ūČēÓŅĻĀĶ¼ĖłŹ¾”£Ķ¼ÖŠaĪŖH2SµÄĘ½ŗā×Ŗ»ÆĀŹÓėĪĀ¶Č¹ŲĻµĒśĻߣ¬bĒśĻß±ķŹ¾²»Ķ¬ĪĀ¶ČĻĀ·“Ó¦¾¹żĻąĶ¬Ź±¼äĒŅĪ““ļµ½»ÆŃ§Ę½ŗāŹ±H2SµÄ×Ŗ»ÆĀŹ”£Čō985”ꏱ£¬·“Ó¦¾t min“ļµ½Ę½ŗā£¬“ĖŹ±H2SµÄ×Ŗ»ÆĀŹĪŖ40%£¬Ōņ·“Ó¦ĖŁĀŹv(H2)£½ £ØÓĆŗ¬c”¢tµÄ“śŹżŹ½±ķŹ¾£©”£ĒėĖµĆ÷ĖęĪĀ¶ČµÄÉżøߣ¬ĒśĻßbĻņĒśĻßa±Ę½üµÄŌŅņ£ŗ ”£
H2(g)£«1/2S2(g) ŌŚŗćČŻĆܱÕČŻĘ÷ÖŠ£¬æŲÖĘ²»Ķ¬ĪĀ¶Č½ųŠŠH2SµÄ·Ö½āŹµŃé£ŗŅŌH2SµÄĘšŹ¼ÅØ¶Č¾łĪŖc mol”¤L£1²ā¶ØH2SµÄ×Ŗ»ÆĀŹ£¬½į¹ūČēÓŅĻĀĶ¼ĖłŹ¾”£Ķ¼ÖŠaĪŖH2SµÄĘ½ŗā×Ŗ»ÆĀŹÓėĪĀ¶Č¹ŲĻµĒśĻߣ¬bĒśĻß±ķŹ¾²»Ķ¬ĪĀ¶ČĻĀ·“Ó¦¾¹żĻąĶ¬Ź±¼äĒŅĪ““ļµ½»ÆŃ§Ę½ŗāŹ±H2SµÄ×Ŗ»ÆĀŹ”£Čō985”ꏱ£¬·“Ó¦¾t min“ļµ½Ę½ŗā£¬“ĖŹ±H2SµÄ×Ŗ»ÆĀŹĪŖ40%£¬Ōņ·“Ó¦ĖŁĀŹv(H2)£½ £ØÓĆŗ¬c”¢tµÄ“śŹżŹ½±ķŹ¾£©”£ĒėĖµĆ÷ĖęĪĀ¶ČµÄÉżøߣ¬ĒśĻßbĻņĒśĻßa±Ę½üµÄŌŅņ£ŗ ”£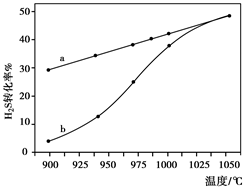
£Ø5£©ÓƶčŠŌµē¼«µē½āĆŗ½¬ŅŗµÄ·½·ØÖĘH2µÄ·“Ó¦ĪŖ£ŗC(s)£«2H2O(l)£½CO2(g)£«2H2(g)ĻÖ½«Ņ»¶ØĮæµÄ1 mol”¤L£1 H2SO4ČÜŅŗŗĶŹŹĮæĆŗ·Ū³ä·Ö»ģŗĻ£¬ÖĘ³Éŗ¬Ģ¼ĮæĪŖ0.02 g”¤mL£1”«0.12g”¤mL£1µÄĆŗ½¬Ņŗ£¬ÖĆÓŚÓŅĶ¼ĖłŹ¾×°ÖĆÖŠ½ųŠŠµē½ā£ØĮ½µē¼«¾łĪŖ¶čŠŌµē¼«£©”£ŌņA¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£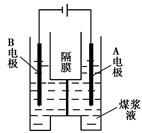
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com