
�±���Ԫ�����ڱ���һ���֣��ش������й����⣺����дԪ�ط��Ż�ѧʽ��
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | �� | �� | ||
| 4 | �� | �� |
(1)д������Ԫ�ط��ţ�
�� ,�� ��
(2)����ЩԪ���У�����õĽ���Ԫ���� ������õķǽ���Ԫ���� ��
��3������ЩԪ���е�����������Ӧ��ˮ�����У�������ǿ���� ��������ǿ���� �������Ե����������� ��
��4������Ԫ�آ۵����ʵ���ɫ�� ���䵥�ʵı��淽����
��5���ڢ�����У���ѧ���ʽϻ��õ��� �������û�ѧʵ��֤���������û�ѧ����ʽ˵����
��
�ڢ�����У���ѧ���ʻ��õ��� �������û�ѧʵ��֤���������û�ѧ����ʽ˵����
��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ������������Ӧԭ���Ľ��͡����۾���ȷ��һ����
| ���������� | ���ͻ���� |
| A�������ھƾ��ƻ����ϼ��ȣ��ۻ��������� | �������ۻ��������������� |
| B����ʢ��0.005mol/L FeCl3��Һ���Թ��м���5mL 0.01mol/LKSCN��Һ����Һ�ʺ�ɫ���ټ���5�α���FeCl3��Һ����ɫ���� | ����Ӧ���Ũ�ȣ���ѧƽ��������Ӧ�����ƶ� |
| C���������Һ�м���ϡ���ᣬˮԡ���ȣ�һ��ʱ����ټ������Ƶ�������ͭ����Һ�����ȣ�δ����ɫ���� | ����δˮ�� |
| D��ȡij��Һ���������������ữ�����ᱵ��Һ�����ְ�ɫ���� | ����Һ��һ�����д�����SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪������Ԫ�ص�����aAm+ ��bB n +��cCm- ��dD n - (m��n)��������ͬ�ĵ��Ӳ�ṹ�� ������������ȷ����
A��ԭ�Ӱ뾶�� A��B��C��D B��ԭ�������� b��a��d��c
C�����Ӱ뾶�� D��C��B��A D��a-d=n+m
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и��������У���ѧ�����Ͳ�ͬ����
A��NaCl��HNO3 B��H2O��NH3 C��CaF2��CsCl D��SiCl4��CH4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��A��B��C���ֶ�����Ԫ�أ���֪AԪ�ص�ԭ��������������������Ӳ�����BԪ�ص�ԭ������������������Ӳ�����2����CԪ�ص�ԭ������������������Ӳ�����3������������Ԫ����ɵĻ�����Ļ�ѧʽ��������
A��A3BC4 B��A2(BC4)3 C��A2BC3 D��ABC4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ú���������г��õ�ȼ��,��ȡˮú���ķ�ӦΪC(s)+H2O(g) CO(g)+H2(g)���÷�Ӧ��һ�ݻ��ɱ���ܱ������н���ʱ,�ı�����������Է�Ӧ���ʲ���Ӱ�����
CO(g)+H2(g)���÷�Ӧ��һ�ݻ��ɱ���ܱ������н���ʱ,�ı�����������Է�Ӧ���ʲ���Ӱ�����
������������̼���ڽ������������Сһ�롡�۱����������,����N2ʹ��ϵѹǿ���ܱ���ѹǿ����,����N2ʹ��ϵ�������
A.�٢� B.�ڢ� C.�ڢ� D.�٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʳ�ð״�(����Ũ��ԼΪ1 mol/L)��������ʵ�飬��֤������Ϊ������ʵ���
A���״��е���ʯ����Һ�ʺ�ɫ������ B���״��붹�����г�������
C�����ǽ����ڰ״���������ų����� D��pH��ֽ��ʾ�����pHΪ2��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧ��ʵ�鱨���м�¼�������ݣ�������ȷ�ģ� ��
A���� 25mL ��Ͳ��ȡ 18.63mL ����
B����������ƽ��ȡ 12.15��ʳ��
C���ñ��� NaOH ��Һ�ζ�δ֪Ũ�ȵ����ᣬ��ȥ NaOH ��Һ32.30mL
D���� pH ��ֽ���ij��Һ pH Ϊ 5.0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����л���������ȷ����( )
A��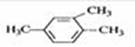 1��3��4-���ױ� B��
1��3��4-���ױ� B�� 2-��-2-�ȱ���
2-��-2-�ȱ���
C��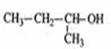 2-��-1-���� D��
2-��-1-���� D��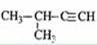 2-��-3-��Ȳ
2-��-3-��Ȳ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com