
£Ø7·Ö£©ČēĶ¼ĖłŹ¾£¬µ±¹Ų±Õ·§ĆÅKŹ±£¬Ļņ¼×ÖŠ³äČė1.5 mol A”¢3.5 mol B£¬ĻņŅŅÖŠ³äČė3 mol A”¢7 mol B£¬ĘšŹ¼Ź±£¬¼×”¢ŅŅĢå»ż¾łĪŖVL”£ŌŚĻąĶ¬ĪĀ¶ČŗĶÓŠ“߻ƼĮ“ęŌŚµÄĢõ¼žĻĀ£¬Į½ČŻĘ÷ÖŠø÷×Ō·¢ÉśĻĀĮŠ·“Ó¦£ŗ3A(g)£«2B(g) 2C(g)£«4D(g)£»¦¤H<0“ļµ½Ę½ŗā£Ø¢ń£©Ź±£¬V(ŅŅ)=1.21V L”£Ēė»Ų“š£ŗ
2C(g)£«4D(g)£»¦¤H<0“ļµ½Ę½ŗā£Ø¢ń£©Ź±£¬V(ŅŅ)=1.21V L”£Ēė»Ų“š£ŗ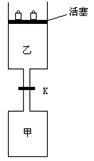
£Ø1£©ŅŅÖŠBµÄ×Ŗ»ÆĀŹĪŖ ”£
£Ø2£©¼×ÖŠDŗĶŅŅÖŠCµÄĪļÖŹµÄĮæ±Č½Ļ£ŗ £ØĢī”°ĻąµČ”±”¢”°Ē°Õß“ó”±”¢”°ŗóÕß“ó”±£©”£
£Ø3£©“ņæŖK£¬¹żŅ»¶ĪŹ±¼äÖŲŠĀ“ļĘ½ŗā£Ø¢ņ£©Ź±£¬ŅŅµÄĢå»żĪŖ £ØÓĆŗ¬VµÄ“śŹżŹ½±ķŹ¾£¬Į¬ĶعÜÖŠĘųĢåĢå»żŗöĀŌ²»¼Ę£©”£
£Ø1£©60ØG£Ø2·Ö£©
£Ø2£©Ē°Õß“ó£Ø2·Ö£©
£Ø3£©0.815V£Ø3·Ö£©
½āĪöŹŌĢā·ÖĪö£ŗ
£Ø1£©ĄūÓĆ·½³ĢŹ½½ųŠŠ¼ĘĖć£ŗ
3A(g)£«2B(g) 2C(g)£«4D(g)
2C(g)£«4D(g)
n£Ø³õ£© 3mol 7mol
¦¤n 9a mol 6a mol 6a mol 12a mol
n£ØÄ©£©(3-9a) mol (7-6a)mol 6a mol 12a mol
ĖłŅŌ£ŗ(3-9a+7-6a+6a+12a)/10=12.1£¬a="0.7" £¬ŌņBµÄ×Ŗ»ÆĀŹĪŖ 6a/7=60%
£Ø2£©×Ŗøł¾ŻµČŠ§Ę½ŗāµÄŌŌņ£¬ŅŅÖŠ·“Ó¦ĪļµÄĮæĪŖ¼×ÖŠµÄĮ½±¶£¬µ«ŹĒŅŅµÄĘ½ŗāדĢ¬ÖŠø÷ĪļÖŹµÄĮæŠ”ÓŚ¼×µÄĮ½±¶£¬²¢ĒŅŅŅÖŠ¼ÓŃ¹Ź¹Ę½ŗāÄęŅĘ¶Æ£¬ĖłŅŌŅŅÖŠCµÄĪļÖŹµÄĮæ±Č¼×ÖŠDµÄĪļÖŹµÄĮ抔”£
£Ø3£©¼×ŅŅ»ģŗĻŗó£¬ÓėŅŅµÄĘ½ŗāדĢ¬¢ńĪŖµČŠ§Ę½ŗā”£ŅŌ60%µÄ×Ŗ»ÆĀŹĄ“¼ĘĖć¼×Ź£ÓąµÄĘųĢåĢå»ż”£ŌņŅŅµÄĢå»żĪŖ0.815V”£
æ¼µć£ŗ»ÆŃ§Ę½ŗāדĢ¬µÄ¼ĘĖć”£µČŠ§Ę½ŗāŌĄķ
µćĘĄ£ŗ±¾ĢāŅŌ³£¼ūµÄ»īČū×öÄ£ŠĶ£¬ÅäŅŌ¼ņµ„µÄ»Æѧ¼ĘĖć£¬×īŗóŅ»æÕŠč¶ŌµČŠ§Ę½ŗā½ųŠŠĄķ½ā”£”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ČēĶ¼ĖłŹ¾£¬µ±¹Ų±Õ·§ĆÅKŹ±£¬Ļņ¼×ÖŠ³äČė1.5mol A”¢3.5mol B£¬ĻņŅŅÖŠ³äČė3mol A”¢7mol B£¬ĘšŹ¼Ź±£¬¼×”¢ŅŅĢå»ż¾łĪŖVL£®ŌŚĻąĶ¬ĪĀ¶ČŗĶÓŠ“߻ƼĮ“ęŌŚµÄĢõ¼žĻĀ£¬Į½ČŻĘ÷ÖŠø÷×Ō·¢ÉśĻĀĮŠ·“Ó¦£ŗ
ČēĶ¼ĖłŹ¾£¬µ±¹Ų±Õ·§ĆÅKŹ±£¬Ļņ¼×ÖŠ³äČė1.5mol A”¢3.5mol B£¬ĻņŅŅÖŠ³äČė3mol A”¢7mol B£¬ĘšŹ¼Ź±£¬¼×”¢ŅŅĢå»ż¾łĪŖVL£®ŌŚĻąĶ¬ĪĀ¶ČŗĶÓŠ“߻ƼĮ“ęŌŚµÄĢõ¼žĻĀ£¬Į½ČŻĘ÷ÖŠø÷×Ō·¢ÉśĻĀĮŠ·“Ó¦£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ČēĶ¼ĖłŹ¾£¬µ±¹Ų±Õ·§ĆÅKŹ±£¬Ļņ¼×ÖŠ³äČė1.5 mol A”¢3.5 mol B£¬ĻņŅŅÖŠ³äČė3 mol A”¢7 mol B£¬ĘšŹ¼Ź±£¬¼×”¢ŅŅĢå»ż¾łĪŖVL”£ŌŚĻąĶ¬ĪĀ¶ČŗĶÓŠ“߻ƼĮ“ęŌŚµÄĢõ¼žĻĀ£¬Į½ČŻĘ÷ÖŠø÷×Ō·¢ÉśĻĀĮŠ·“Ó¦£ŗ3A(g)£«2B(g) C(g)£«2D(g) ¦¤H<0£»“ļµ½Ę½ŗā£Ø¢ń£©Ź±£¬V(ŅŅ)=0.86V L”£Ēė»Ų“š£ŗ
C(g)£«2D(g) ¦¤H<0£»“ļµ½Ę½ŗā£Ø¢ń£©Ź±£¬V(ŅŅ)=0.86V L”£Ēė»Ų“š£ŗ
£Ø1£©ŅŅÖŠBµÄ×Ŗ»ÆĀŹĪŖ
£Ø2£©¼×ÖŠDŗĶŅŅÖŠCµÄĪļÖŹµÄĮæ±Č½Ļ£ŗ £ØĢī”°ĻąµČ”±”¢”°Ē°Õß“ó”±”¢”°ŗóÕß“ó”±£©£»
£Ø3£©“ņæŖK£¬¹żŅ»¶ĪŹ±¼äÖŲŠĀ“ļĘ½ŗā£Ø¢ņ£©Ź±£¬ŅŅµÄĢå»żĪŖ £ØÓĆŗ¬VµÄ“śŹżŹ½±ķŹ¾£¬Į¬ĶعÜÖŠĘųĢåĢå»żŗöĀŌ²»¼Ę”££©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģÉĀĪ÷Ź”½»“óø½ÖŠøßČżµŚĖÄ“ĪÕļ¶Ļ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
ČēĶ¼ĖłŹ¾£¬µ±¹Ų±Õ·§ĆÅKŹ±£¬Ļņ¼×ÖŠ³äČė1.5 mol A”¢3.5 mol B£¬ĻņŅŅÖŠ³äČė3 mol A”¢7 mol B£¬ĘšŹ¼Ź±£¬¼×”¢ŅŅĢå»ż¾łĪŖV L”£ŌŚĻąĶ¬ĪĀ¶ČŗĶÓŠ“߻ƼĮ“ęŌŚµÄĢõ¼žĻĀ£¬Į½ČŻĘ÷ÖŠø÷×Ō·¢ÉśĻĀĮŠ·“Ó¦£ŗ3A(g)+ 2B(g) C(g)
C(g) £«2D(g)£»¦¤H<0“ļµ½Ę½ŗā£Ø¢ń£©Ź±£¬V(ŅŅ)="0.86V" L”£Ēė»Ų“š£ŗ
£«2D(g)£»¦¤H<0“ļµ½Ę½ŗā£Ø¢ń£©Ź±£¬V(ŅŅ)="0.86V" L”£Ēė»Ų“š£ŗ
£Ø1£©ŅŅÖŠBµÄ×Ŗ»ÆĀŹĪŖ £»
£Ø2£©¼×ÖŠDŗĶŅŅÖŠCµÄĪļÖŹµÄĮæ±Č½Ļ£ŗ £ØĢī”°ĻąµČ”±”¢”°Ē°Õß“ó”±”¢”°ŗóÕß“ó”±£©£»
£Ø3£©“ņæŖK£¬¹żŅ»¶ĪŹ±¼äÖŲŠĀ“ļĘ½ŗā£Ø¢ņ£©Ź±£¬ŅŅµÄĢå»żĪŖ £ØÓĆŗ¬VµÄ“śŹżŹ½±ķŹ¾£¬Į¬ĶعÜÖŠĘųĢåĢå»żŗöĀŌ²»¼Ę£©”£zxxk
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğÕć½Ź”½š»ŖŅ»ÖŠø߶žĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗ¼ĘĖćĢā
ČēĶ¼ĖłŹ¾£¬µ±¹Ų±Õ·§ĆÅKŹ±£¬Ļņ¼×ÖŠ³äČė1.5 mol A”¢3.5 mol B£¬ĻņŅŅÖŠ³äČė3 mol A”¢7 mol B£¬ĘšŹ¼Ź±£¬¼×”¢ŅŅĢå»ż¾łĪŖV L”£ŌŚĻąĶ¬ĪĀ¶ČŗĶÓŠ“߻ƼĮ“ęŌŚµÄĢõ¼žĻĀ£¬Į½ČŻĘ÷ÖŠø÷×Ō·¢ÉśĻĀĮŠ·“Ó¦£ŗ3A(g)£«2B(g) C(g)£«2D(g) ¦¤H<0£»“ļµ½Ę½ŗā£Ø¢ń£©Ź±£¬V(ŅŅ)="0.86V" L”£Ēė»Ų“š£ŗ
C(g)£«2D(g) ¦¤H<0£»“ļµ½Ę½ŗā£Ø¢ń£©Ź±£¬V(ŅŅ)="0.86V" L”£Ēė»Ų“š£ŗ
£Ø1£©ŅŅÖŠBµÄ×Ŗ»ÆĀŹĪŖ
£Ø2£©¼×ÖŠDŗĶŅŅÖŠCµÄĪļÖŹµÄĮæ±Č½Ļ£ŗ £ØĢī”°ĻąµČ”±”¢”°Ē°Õß“ó”±”¢”°ŗóÕß“ó”±£©£»
£Ø3£©“ņæŖK£¬¹żŅ»¶ĪŹ±¼äÖŲŠĀ“ļĘ½ŗā£Ø¢ņ£©Ź±£¬ŅŅµÄĢå»żĪŖ £ØÓĆŗ¬VµÄ“śŹżŹ½±ķŹ¾£¬Į¬ĶعÜÖŠĘųĢåĢå»żŗöĀŌ²»¼Ę”££©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğÉĀĪ÷Ź”øßČżµŚĖÄ“ĪÕļ¶Ļ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
ČēĶ¼ĖłŹ¾£¬µ±¹Ų±Õ·§ĆÅKŹ±£¬Ļņ¼×ÖŠ³äČė1.5 mol A”¢3.5 mol B£¬ĻņŅŅÖŠ³äČė3 mol A”¢7 mol B£¬ĘšŹ¼Ź±£¬¼×”¢ŅŅĢå»ż¾łĪŖV
L”£ŌŚĻąĶ¬ĪĀ¶ČŗĶÓŠ“߻ƼĮ“ęŌŚµÄĢõ¼žĻĀ£¬Į½ČŻĘ÷ÖŠø÷×Ō·¢ÉśĻĀĮŠ·“Ó¦£ŗ3A(g)+ 2B(g) C(g)
C(g) £«2D(g)£»¦¤H<0“ļµ½Ę½ŗā£Ø¢ń£©Ź±£¬V(ŅŅ)=0.86V L”£Ēė»Ų“š£ŗ
£«2D(g)£»¦¤H<0“ļµ½Ę½ŗā£Ø¢ń£©Ź±£¬V(ŅŅ)=0.86V L”£Ēė»Ų“š£ŗ

£Ø1£©ŅŅÖŠBµÄ×Ŗ»ÆĀŹĪŖ £»
£Ø2£©¼×ÖŠDŗĶŅŅÖŠCµÄĪļÖŹµÄĮæ±Č½Ļ£ŗ £ØĢī”°ĻąµČ”±”¢”°Ē°Õß“ó”±”¢”°ŗóÕß“ó”±£©£»
£Ø3£©“ņæŖK£¬¹żŅ»¶ĪŹ±¼äÖŲŠĀ“ļĘ½ŗā£Ø¢ņ£©Ź±£¬ŅŅµÄĢå»żĪŖ £ØÓĆŗ¬VµÄ“śŹżŹ½±ķŹ¾£¬Į¬ĶعÜÖŠĘųĢåĢå»żŗöĀŌ²»¼Ę£©”£zxxk
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com