
����Ŀ�������仯�����������ҵ������������ҪӦ�á���ش��������⣺
(1)��ͼ��N2(g)��H2(g)��NH3(g)֮��ת����������ϵͼ����
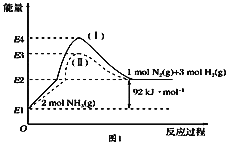
��N2(g)��H2(g)��Ӧ����NH3(g)���Ȼ�ѧ����ʽΪ___________________.
�ڹ���(��)����(��)�ķ�Ӧ��________(���ͬ����ͬ��).
��ij�¶��£���1 L���º��������г���1molN2��3 mol H2����������Ӧ��10 min�ﵽƽ�⣬��ʱ������ѹǿ��Ϊԭ����7/8.
a.�ù��̵�ƽ�ⳣ���ı���ʽΪ____________.
b.N2��ƽ��ת����Ϊ________.
c.��ʱ�����������¶Ⱥ�������䣬�������ټ���2.25 molN2��0.5 mol NH3����ƽ��________(�����������)�ƶ�.
(2)��NH3�������������������Ⱦ����֪��
��Ӧ��4NH3(g)��3O2(g)![]() 2N2(g)��6H2O(g) ��H1��a kJ��mol��1 ƽ�ⳣ��ΪK1
2N2(g)��6H2O(g) ��H1��a kJ��mol��1 ƽ�ⳣ��ΪK1
��Ӧ��N2(g)��O2(g)![]() 2NO(g) ��H2��b kJ��mol��1 ƽ�ⳣ��ΪK2
2NO(g) ��H2��b kJ��mol��1 ƽ�ⳣ��ΪK2
��Ӧ��4NH3(g)��6NO(g)![]() 5N2(g)��6H2O(g) ��H3��c kJ��mol��1 ƽ�ⳣ��ΪK3
5N2(g)��6H2O(g) ��H3��c kJ��mol��1 ƽ�ⳣ��ΪK3
��Ӧ���е�b��_____(�ú�a��c�Ĵ���ʽ��ʾ)��K3=_____(��K1��K2��ʾ).��Ӧ���еĦ�S______(�>����<������)0.
(3)�ں��ݵ��ܱ����У�����һ������NH3��NO����������Ӧ��ò�ͬ�¶��·�Ӧ��ϵ��NH3��ת����(��)��ѹǿp�Ĺ�ϵ��ͼ��ʾ��
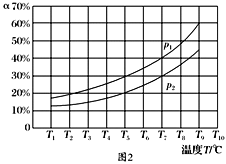
�ٷ�����p1________p2.(�>����<������)
�����������У�������Ϊ�жϷ�Ӧ���Ѿ��ﵽƽ��״̬�ı�־����________(�����).
a��N2��Ũ�Ȳ��ٸı� b������6 mol N��H����ͬʱ����6 mol H��O���γ�
c��������ѹǿ���ٱ仯 d�����������ܶȱ��ֲ���
���𰸡�N2(g)��3H2(g)![]() 2NH3(g) ��H����92 kJ��mol��1 ��ͬ K=c2(NH3)/��c(N2)c3(H2)�� 25% �� (a-c)/3
2NH3(g) ��H����92 kJ��mol��1 ��ͬ K=c2(NH3)/��c(N2)c3(H2)�� 25% �� (a-c)/3 ![]() > < bd
> < bd
��������
��1���پ�ͼ��֪2molNH3�ֽ�õ�1molN2��3molH2������92kJ/mol�����������N2(g)��H2(g)��Ӧ����NH3(g)���Ȼ�ѧ����ʽΪN2(g)��3H2(g) ![]() 2NH3(g) ��H����92 kJ��mol��1��
2NH3(g) ��H����92 kJ��mol��1��
�ڸ��ݸ�˹���ɣ���Ӧ��ֻ����ʼ״̬������״̬�йأ�������أ�������������ʼ״̬������״̬��ͬ�������Ӧ����ͬ��
�ۿ��Ը�������ʽȥ��⣬��ת��xmol/LN2��
N2(g)��3H2(g) ![]() 2NH3(g)
2NH3(g)
�� 1 3 0
ת x 3x 2x
ƽ 1-x 3-3x 2x
���ݴ�ʱ������ѹǿ��Ϊԭ����7/8������ʽ��![]() ����x=0.25mol/L��
����x=0.25mol/L��
a. K= ![]() ��
��
b. N2��ƽ��ת����Ϊ0.25/1��100��=25����
c. ƽ��ʱc(N2)=0.75mol/L��c(H2)=2.25mol/L��c(NH3)=0.5mol/L��K=![]() =0.029���������ټ���2.25 molN2��0.5 mol NH3����c(N2)=3mol/L��c(H2)=2.25mol/L��c(NH3)=1mol/L��Q=
=0.029���������ټ���2.25 molN2��0.5 mol NH3����c(N2)=3mol/L��c(H2)=2.25mol/L��c(NH3)=1mol/L��Q= ![]() =0.029�����Q=K��ƽ�ⲻ�ƶ���
=0.029�����Q=K��ƽ�ⲻ�ƶ���
��2�����ݸ�˹���ɣ��跴Ӧ�����������ֱ�ΪA��B��C����B=(A-C)/3�����b=(a-c)/3������c=a-3b�����Կ�֪K3=K1/K23������4NH3(g)��6NO(g)![]() 5N2(g)��6H2O(g)��֪�÷�Ӧ�����ʾ�Ϊ���壬�������������������Ҷȱ�ʦ�S>0��
5N2(g)��6H2O(g)��֪�÷�Ӧ�����ʾ�Ϊ���壬�������������������Ҷȱ�ʦ�S>0��
��3������4NH3(g)��6NO(g)![]() 5N2(g)��6H2O(g)��֪�÷�Ӧ������������ڷ�Ӧǰ��������������¶Ȳ��䣬NH3��ת����(��)Խ��˵��ѹǿԽС�����p1<p2��a��N2��Ũ�Ȳ��ٸı䣬˵�������Ũ�ȶ����䣬���������Ϊ�ж�ƽ��ı�־����ȷ��b������6 mol N��H����ͬʱ����6 mol H��O���γɣ����ݷ�Ӧ�ص㣬���߶���ʾ����Ӧ���ʣ��������c�����ڷ�Ӧǰ������������仯����������ѹǿ���ٱ仯�����ж�ƽ�⣬��ȷ��d�����������ܶȦ�=m/V�����ڸ���ֶ������壬��Ӧǰ�����������������䣬�������㶨��������䣬����ܶ�ʼ���Ǹ���ֵ�����ʴ�ѡbd��
5N2(g)��6H2O(g)��֪�÷�Ӧ������������ڷ�Ӧǰ��������������¶Ȳ��䣬NH3��ת����(��)Խ��˵��ѹǿԽС�����p1<p2��a��N2��Ũ�Ȳ��ٸı䣬˵�������Ũ�ȶ����䣬���������Ϊ�ж�ƽ��ı�־����ȷ��b������6 mol N��H����ͬʱ����6 mol H��O���γɣ����ݷ�Ӧ�ص㣬���߶���ʾ����Ӧ���ʣ��������c�����ڷ�Ӧǰ������������仯����������ѹǿ���ٱ仯�����ж�ƽ�⣬��ȷ��d�����������ܶȦ�=m/V�����ڸ���ֶ������壬��Ӧǰ�����������������䣬�������㶨��������䣬����ܶ�ʼ���Ǹ���ֵ�����ʴ�ѡbd��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�������������Ͻ���300mL 2mol/L������ȫ��Ӧ����3.36LNO(���)���������Ρ����εȣ�����Ӧ�����Һ�м���3mol/L��NaOH��Һ��ʹ������Ԫ����ȫ����������������NaOH��Һ������ǣ� ��
A. 450mL B. 150mL C. 200mL D. ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ȤС���Ʊ�һ��������������.ȡ3mL��ˮ�Ҵ�,2mLŨ����,2mL���������ʵ�飬��5mL����̼������Һ�ռ�����.

I.ʵ��װ����ͼ��ʾ
��1���Ʊ����������Ļ�ѧ����ʽΪ____________��
��2��Ũ�����������_________��
��3������װ�û���ѡ����ͼ�е�_______��������ţ�.

��.��ͬѧ�ú��з�̪�ı���̼������Һ���ʼ��ԣ��ռ�����������ֺ�ɫѸ����ȥ.
��ͬѧ��Ϊ�������������к���̼����.��ͬѧͨ���������ϲ���������ʵ�飬֤����ͬѧ���Ʋ��Ǵ���ġ�
��֪����̪������ˮ���������л��ܼ�����̪�Լ��Ƿ�̪���Ҵ���Һ.
ʵ��i��ȡ����²���ɫҺ�壬�ֳ����ݣ��ֱ��������ʵ��
��� | ʵ����� | ʵ������ | ���� |
1 | �μӼ�����̪�Լ� | ��Һ______������������������������ | ̼���Ʋ�δ��������ȫ�кͣ����д���ʣ�� |
2 | ����������Һ | �д������ݲ� |
ʵ��ii.ȡ����ϲ�Һ�壬����____��Һ�������ֳ���dz��ɫ�����÷ֲ���ɫ��ʧ��
ʵ��iii��ȡ5mL����̼������Һ�����뼸�η�̪�Լ����ټ���3mL�����������������ᣩ����Һ�ȱ�죬���ɫ��ʧ���ش���������
��4���������ʵ�飺��________����________��
��5�����ʵ��ii��ʵ��iii�����ɵó��Ľ�����__________��
��6��ʵ��iii��ʵ��Ŀ����___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ݱ�������ѧ���Ѿ����Ƴ���������IJ��ϡ���̼ĤƬ������ֻ��һ��ͷ���Ķ�ʮ���֮һ����ͼ��ʾ������̼ĤƬ��״��䳲������̼ԭ�ӹ��ɵ������ε�Ԫ������չ���ɣ������й�̼ĤƬ��˵���У���ȷ���ǣ� ��
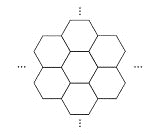
A. ̼ĤƬ����һ�����͵Ļ�����
B. ̼ĤƬ��ʯī�Ľṹ��ͬ
C. ̼ĤƬ��C60��Ϊͬ��������
D. ̼ĤƬ����������ȫȼ�յIJ����̼����������ȫȼ�յIJ��ﲻͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ϩ����������ȩ����Ƴ���ͼ��ʾ��ȼ�ϵ�أ������Ʊ���ȩ��ͬʱ��õ��ܣ����ܷ�ӦΪ2CH2=CH2��O2===2CH3CHO.�����й�˵������ȷ����
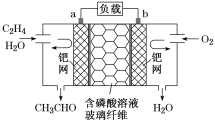
A. ÿ��0.1molO2��Ӧ����Ǩ��H��0.4mol
B. ������ӦʽΪCH2=CH2��2e����H2O=CH3CHO��2H��
C. �����ƶ����缫a��������Һ���缫b
D. �õ��Ϊ���ɳ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾΪ�ӹ��������з���X�����ַ���������ݷ���1�ͷ���2ָ������˵���к�������(����)

A. ����ѡ�÷���1����̼�������к��е��Ȼ��
B. ����1�еIJ�����Ӧ�þ��е������������ӷ�
C. ����2�м�����Լ�һ���ܹ����X������ʷ�����ѧ��Ӧ
D. ����2�м���NaOH��Һ���Է����SiO2��Fe2O3������е�Fe2O3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����̬Kԭ���У��������ռ������ܲ�ķ�����____��ռ�ݸ��ܲ���ӵĵ���������ͼ��״Ϊ______��
��2����̬Geԭ�ӵĺ�������Ų�ʽΪ[Ar]_______����______��δ�ɶԵ��ӡ�
��3��д����̬Asԭ�ӵĺ�������Ų�ʽ____������Ԫ�������ɣ�ԭ�Ӱ뾶Ga___As����һ������Ga____As��������ڡ���С�ڡ���
��4��Zn2+��̬��������Ų�ʽΪ____________________��
��5����ԭ�Ӽ۲���ӵĹ������ʽ�������Ų�ͼ��Ϊ_____________��
��6��CH4��CO2����������Ԫ�ص縺�Դ�С�����˳��Ϊ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и����л��������У�����������ʲô������ϣ�ֻҪ�����ʵ���һ��������ȫȼ��ʱ���ɵ�ˮ�����������������������������
A. CH4O��C3H4O5B. C3H6��C3H8O
C. C3H8��C4H6D. C2H6��C4H6O2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڲ�ͬ�����·ֱ��÷�Ӧ2SO2+O2![]() 2SO3�Ļ�ѧ��Ӧ���ʣ����б�ʾ�÷�Ӧ���������ǣ� ��
2SO3�Ļ�ѧ��Ӧ���ʣ����б�ʾ�÷�Ӧ���������ǣ� ��
A. v(SO2)��4 mol��L-1��min-1B. v (O2)��3 mol��L-1��min-1
C. v (SO3)="0.1" mol��L-1��s-1D. v (O2)��0.1mol��L-1��s-1
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com