
þ�ڿ�����ȼ�ճ�����MgO�⣬����������Mg3N2��ijУ��ѧ��ȤС���ͬѧ����þ�ڿ�����ȼ�պ�Ĺ���(��������)����ʵ�飬̽������ɡ�
(1)����ͬѧȡһ����ȼ�պ�Ĺ���Ͷ��ˮ�У��õ���һ����ʹʪ��ĺ�ɫʯ����ֽ���������壬������Ļ�ѧʽΪ____________________��˵�������к���Mg3N2�����ɸ�����Ļ�ѧ����ʽΪ________________________________________________________________________
________________________________________________________________________��
(2)����ͬѧΪ�ⶨMg3N2�ĺ���������ͼ��ʾװ�ý���ʵ�飬��ַ�Ӧ���ټ���A������Ũ�����������__________________________________________________________________
______________________________________����A���ȵ�Ŀ����________________________
________________________________________________________________________��
��֪����Ĺ�������Ϊ4.0 g������Cװ������a g��������к�Mg3N2______ g(�ú�a��ʽ�ӱ�ʾ)��
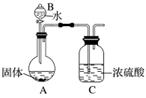
(3)�����е�ͬѧ��Ϊ����ͬѧ�IJⶨ���ƫ�ߣ�������_______________________��
�е�ͬѧ��Ϊ����ͬѧ�IJⶨ���ƫ�ͣ�������________________________________________________________________________
________________________________________________________________________��
����ͬѧ�����˸Ľ������ǽ�����ͬѧʵ���еõ������ܹ�����й��ˡ�ϴ�ӡ���������չ��������أ����������Ϊ4.08 g�����������У�ϴ�ӳ����IJ�����________________________________________________________________________
________________________________________________________________________��
þ�ڿ�����ȼ�պ����ɵĹ�����Mg3N2����������Ϊ______________��
(4)��һ��������뺬þ��ʯ���������ȡ����þ�ķ�������������з������ڵ�ԭ�ϳɱ��ߡ���ĿͶ�ʴ��ܺĸߡ�����Ʒ�����õ����⣬��ԭ���ǽ���þ��ʯ��(������þ)����λ�ϣ��������ա�ˮ�ܡ����ˣ��õ���þ����Һ�����������ղ����İ���д���ù���(NH4)2SO4�뺬þ��ʯ�ۻ������ʱ�Ļ�ѧ��Ӧ����ʽ____________________________________
________________________________________________________________________��
�𰸡�(1)NH3��Mg3N2��6H2O===3Mg(OH)2��2NH3��
(2)�������ɵ�NH3��ʹAװ���з�Ӧ������NH3ȫ�����뵽Cװ���С�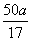
(3)Cװ�õ���������װ�ã�NH3�е�ˮ�����Լ��������е�ˮ���������ܽ���Cװ�ö���Ũ�������գ���ɽ��ƫ�ߡ������в���NH3������װ����û����ȫ��Ũ�������գ��Ӷ���ɽ��ƫ�͡���������еij������ϼ�����ˮ��ǡ����ȫ��û��������ˮȫ���������ظ�����2��3�Ρ�10%
(4)(NH4)2SO4��MgO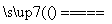 MgSO4��2NH3����H2O
MgSO4��2NH3����H2O
������(1)��ʹʪ��ĺ�ɫʯ����ֽ������������NH3��Mg3N2��ˮ��Ӧ����Mg(OH)2��NH3��(2)Ũ���������Ը������������NH3����A���ȿɴ�ʹNH3�ӷ���ʹ��Ӧ������NH3ȫ����Cװ�����գ����ɹ�ϵʽMg3N2��2NH3�����a g NH3��Ӧ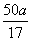 g Mg3N2��(3)Aװ���в�����NH3�Ậ��ˮ������Cװ����ŨH2SO4������NH3��Ҳ����NH3�е�ˮ����������Cװ�ú�δ���Ӹ���������ˮ������װ�ã�����Cװ�����ص�a g�а���ˮ��������������ɲⶨ���ƫ�ߡ���Ӧ������NH3�ڷ�Ӧ������������ѹ��ԭ����ȫ�����ų�������װ��������һ���֣���ɲ�����NH3����
g Mg3N2��(3)Aװ���в�����NH3�Ậ��ˮ������Cװ����ŨH2SO4������NH3��Ҳ����NH3�е�ˮ����������Cװ�ú�δ���Ӹ���������ˮ������װ�ã�����Cװ�����ص�a g�а���ˮ��������������ɲⶨ���ƫ�ߡ���Ӧ������NH3�ڷ�Ӧ������������ѹ��ԭ����ȫ�����ų�������װ��������һ���֣���ɲ�����NH3����
ȫ����Ũ�������գ����Ի���ɲⶨ���ƫ�͡�����ͬѧ����4.08 g������MgO������ԭ������е�MgO��Mg3N2ת������MgO(Mg3N2��3Mg(OH)2��3MgO)����ԭ����������Mg3N2��MgO�����ʵ����ֱ�Ϊx mol��y mol��
��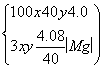
��ã�x��0.004��y��0.09
��ȼ�պ����ɵĹ�����Mg3N2��������Ϊ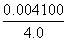 ��100%��10%��
��100%��10%��
(4)��þ��ʯ���к�MgO��MgO��(NH4)2SO4���·�Ӧ����MgSO4��NH3��H2O��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��M��N������Һ�����ⶨ��������Һ�к�������12�����ӣ�Al3����Cl����Na����K����NO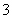 ��OH����Fe2����AlO
��OH����Fe2����AlO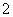 ��CO
��CO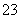 ��NH
��NH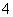 ��SO
��SO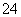 ��H����
��H����
(1)������б�����ʵ��ٵĽ��ۺ�ʵ��ڵ�ʵ�������Լ�����
| ʵ�������Լ����� | ���� |
| ��ȡ����N��Һ�μ����������ᱵ��Һ���������� | |
| �� | ȷ��M��Һ�к���Na��������K�� |
| ����pH��ֽ���M��Һ��pH��ֽ����ɫ |
(2)����(1)�е�ʵ��ش�
NO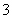 ������________��Һ�У�������________________________________________________________________________��
������________��Һ�У�������________________________________________________________________________��
Cl��������________��Һ�У�������________________________________________________________________________��
(3)����(1)�е�ʵ��ȷ����M��Һ�к��е�����Ϊ________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и�������У��ܳ��֡��Ȳ���������Ȼ��������ܽ⡱�������______________��
����̼������Һ��ͨ�������CO2������NaAlO2��Һ����μ��������ϡ���ᡡ����AlCl3��Һ����μ������ϡ����������Һ�������������Һ����μ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һ�ֵͶ�����Ԫ�أ�������������Ҫ����Ԫ�أ�ʳ�úᵼ�¼����ж�����ʳƷ�����ĺ����������ұ��ͻ���������Σ�������й�����Ԫ�ص�˵����ȷ����(����)
A�����ڿ����в�����������Ϊ�����ʲ�����
B��������������θ�ᷴӦ���������к�θ���ҩ��
C����������������ˮ��ɱ������
D��������麟�����������������ʲ�����ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ɫ����Һ������Ӧ�ų�����������Һ�п��ܺ���Mg2����Cu2����Ba2����H����Ag����SO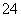 ��SO
��SO ��HCO
��HCO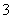 ��OH����NO
��OH����NO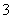 ʮ�������е������֣������ƶ���ȷ����(����)
ʮ�������е������֣������ƶ���ȷ����(����)
A������Һ����Al3������ʱ����Һ�п��ܴ��ڣ�SO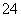 ��NO
��NO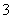 ��H����Mg2��
��H����Mg2��
B������Һ����Al3������ʱ����Һ��һ�����ڣ�H����SO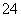 �����ܴ���Mg2��
�����ܴ���Mg2��
C������Һ����AlO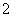 ����ʱ����Һ��һ�����ڣ�OH����Ba2����NO
����ʱ����Һ��һ�����ڣ�OH����Ba2����NO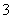
D������Һ����AlO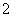 ����ʱ����Һ�п��ܴ��ڣ�OH����Ba2����NO
����ʱ����Һ�п��ܴ��ڣ�OH����Ba2����NO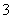 ��SO
��SO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������������(����)
A�����෴��ɵ�����֮����������Ϊ���Ӽ�
B������Ԫ����ǽ���Ԫ�ػ���ʱ����һ���γ����Ӽ�
C��ijԪ�ص�ԭ�������ֻ��һ�����ӣ�����±�ؽ��ʱ���γɵĻ�ѧ����һ�������Ӽ�
D���ǽ���ԭ�Ӽ�����γ����Ӽ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ǿ��������۵����������йأ��۵�����Խ�࣬������Խǿ������������ӵİ뾶��СҲ�йأ����������Ӱ뾶Խ������Խ�����ݴ��жϣ����н����۵������ߵ���(����)
A��Li��Na��K B��Na��Mg��Al
C��Li��Be��Mg D��Li��Na��Mg
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������(��������ԭ��)��һ����˵���ɼ��Է�����ɵ����������ڼ��Է�����ɵ��ܼ����Ǽ��Է�����ɵ����������ڷǼ��Է�����ɵ��ܼ���������ʵ�п����á��������ܡ�ԭ��˵������(����)
��HCl������ˮ����I2������ˮ����Cl2������ˮ
��NH3������ˮ
A���٢� B���ڢ�
C���٢� D���ۢ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com